Use data from Appendix D to determine (a) r H, r S, and r
Question:
Use data from Appendix D to determine
(a) ΔrH°, ΔrS°, and ΔrG° at 298 K and
(b) K at 875 K for the water gas shift reaction, used commercially to produce H2(g): CO(g) + H2O(g) Δ CO2(g) + H2(g).
Transcribed Image Text:
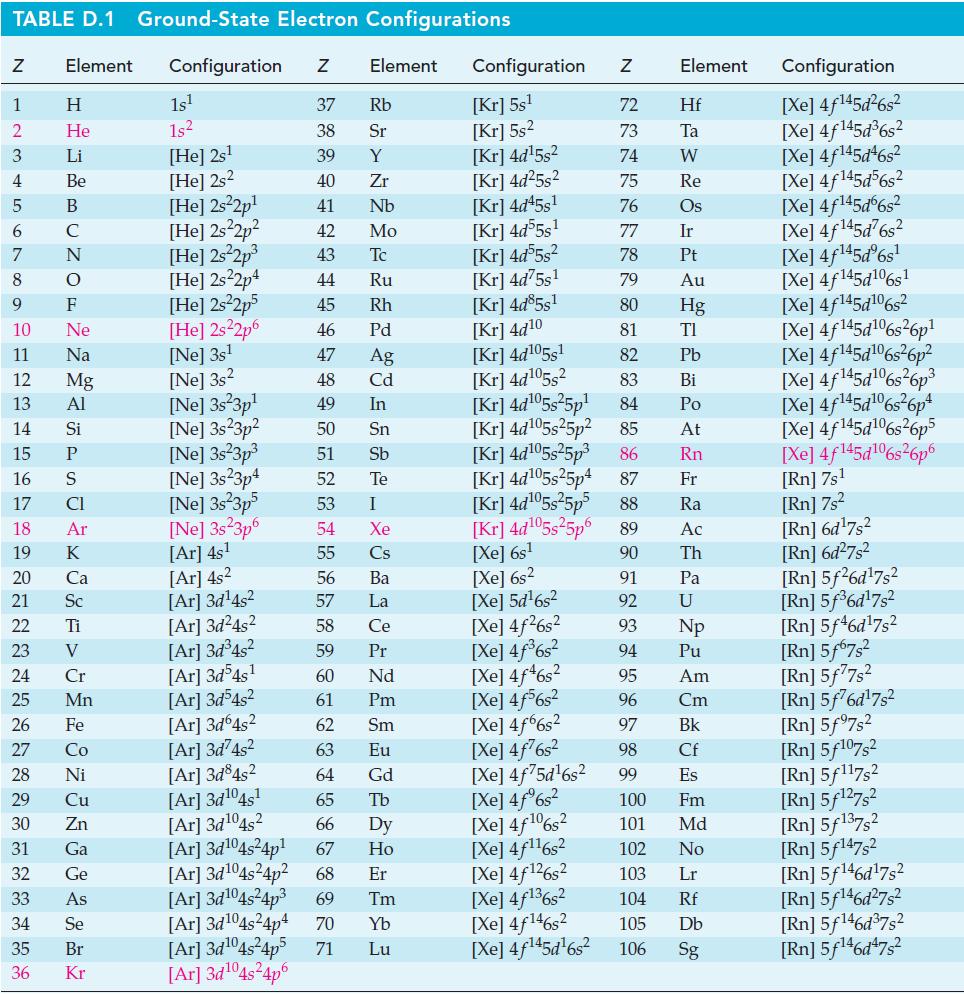
TABLE D.1 Ground-State Electron Configurations Element Configuration Z Z 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 24 25 27 HIGÅ LUZONS JY SE> 0 ≤ 2 3 2 3 5 3 3 4 8 5 2 Η 29 He 30 Li 31 Be 32 B 33 C F Ne Na 20 Ca Mg 21 Sc Al 22 Ti Si 23 V P CI Ar K 26 Fe 28 Ni Cr Mn Co Cu Zn Ga Ge As 34 Se 35 Br 36 Kr 1s¹ 1s² [He] 2s¹ [He] 2s2 [He] 2s²2p¹ [He] 2s²2p² [He] 2s²2p³ [He] 2s22p4 [HE] 2s²2p5 [He] 2s²2p6 [Ne] 3s¹ [Ne] 3s2 [Ne] 3s23p¹ [Ne] 3s23p² [Ne] 3s²3p³ [Ne] 3s23p4 [Ne] 3s 3p5 [Ne] 3s23p6 [Ar] 4s¹ [Ar] 4s² [Ar] 3d¹4s² Element [Ar] 3d¹04s²4p6 37 Rb 38 Sr 39 Y 40 Zr 41 Nb 42 Mo 43 Tc 44 Ru 45 Rh 46 Pd 47 Ag 48 Cd 49 In 50 Sn 51 Sb 52 Te 53 54 55 56 57 58 59 60 61 62 63 64 I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd [Ar]3d²4s² [Ar] 3d³4s² [Ar]3d54s¹ [Ar] 3d³4s² [Ar] 3d64s² [Ar] 3d74s² [Ar] 3d845² [Ar] 3d¹04s¹ [Ar] 3d¹04s2 65 Tb 66 67 [Ar] 3d¹04s²4p¹ [Ar] 3d¹04s²4p² 68 Dy Ho Er [Ar] 3d¹04s²4p³ 69 Tm [Ar] 3d¹04s²4p4 70 Yb [Ar] 3d¹04s²4p5 71 Lu Configuration Z [Kr] 5s¹ [Kr] 5s² [kr] 4d¹5s² [Kr] 4d²5s² [Kr] 4d45s¹ [kr] 4d55s¹ [Kr] 4d55s² [Kr] 4d75s¹ [Kr] 4d85s1 [Kr] 4d10 [Kr] 4d105s1 [Kr] 4d¹05s² [Kr] 4d¹05s²5p¹ [kr] 4d¹05s25p² [kr] 4d¹05s²5p³ [Kr] 4d¹05s25p4 [Xe] 6s² [Xe] 5d¹6s² [Xe] 4f²6s² [Xe] 4f³6s² [Xe] 4f46s2 [Xe] 4f6s2 [Xe] 4f6s2 [Xe] 4f²6s² [Xe] 4f75d¹6s² [Xe] 4f%s2 [Xe] 4f106s2 [Xe] 4f¹¹6s² NRNKERKR [Xe] 4f126s2 [Xe] 4f136s2 [Xe] 4f146s2 [Xe] 4f¹45d¹6s² 72 Hf 73 Ta W 74 75 Re 76 Os 77 Element 78 79 80 81 82 83 84 85 86 87 Fr [kr] 4d¹05s²5p5 88 Ra [Kr] 4d¹05s²5p6 89 Ac [Xe] 6s¹ 90 Th 91 92 93 94 95 96 97 98 99 Ir Pt Au Hg TI Pb Bi Po At Rn Pa U Np Pu Am Cm Bk Cf Es 100 Fm 101 Md 102 No 103 Lr 104 Rf 105 Db 106 Sg Configuration [Xe] 4f¹45d²6s² [Xe] 4f145d³6s² [Xe] 4f145d46s2 [Xe] 4f145d56s2 [Xe] 4f145d6s2 [Xe] 4f¹45d²6s² [Xe] 4f¹45dº6s¹ [Xe] 4f145d106s1 [Xe] 4f145d106s2 [Xe] 4f145d6s26p* [Xe] 4f145d106s36p? [Xe] 4f145d16s?6p3 [Xe] 4f145d6s®6p* [Xe] 4f145d16s26p5 [Xe] 4f145d106s26p6 [Rn] 7s¹ [Rn] 7s² [Rn] 6d¹7s² [Rn] 6d²7s² [Rn] 5f26d¹7s² [Rn] 5f³6d¹7s² [Rn] 5f46d¹7s2 [Rn] 5f67s² [Rn] 5f77s² [Rn] 5f76d¹7s² [Rn] 5f97s2 [Rn] 5f107,2 [Rn] 5f117s2 [Rn] 5f¹27s² [Rn] 5f137,2 [Rn] 5f147s2 Rn] 5f¹46d¹7s² [Rn] 5f¹46d²7s² [Rn] 5f¹46d³7s2 [Rn] 5f¹46d¹7s²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a rH rS and rG at 298 K for the water gas shift reaction Using the d...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
In Example 13-3, we dealt with vap H and vap S for water at 100 C. (a) Use data from Appendix D to determine values for these two quantities at 25 C. (b) From your knowledge of the structure of...
-
Use data from Appendix D to establish for the reaction 2 N 2 O 4 (g) + O 2 (g) 2 N 2 O 5 (g): (a) r G at 298 K for the reaction as written; (b) K at 298 K. TABLE D.1 Ground-State Electron...
-
Use data from Appendix D to determine values at 298 K of and K for the following reactions. (The equations are not balanced.) (a) HCl(g) + O(g) (b) FeO3(s) + H(g) (c) Ag(aq) + SO4 (aq) HO(g) + Cl(g)...
-
A particle is thrown vertically upwards into the air. Its height s (in m) above the ground after time t (in seconds) is given by s = 25t 5t 2 (a) What height does the particle reach? (b)What is its...
-
Lager Dental Clinic is a medium-sized dental service specializing in family dental care. The clinic is currently preparing the master budget for the first 2 quarters of 2014. All that remains in this...
-
In Appendix 16A.1, we illustrate the calculation of a standard error for the marginal effect in a probit model of transportation, Example 16.4. In the appendix, the calculation is for the marginal...
-
Define, using context-based hierarchy, the main contexts and subcontexts of the mission context that describes your particular driving pattern from home to work each day.
-
Better Products, Inc., manufactures three products on two machines. In a typical week, 40 hours are available on each machine. The profit contribution and production time in hours per unit are as...
-
I need the answer ASAP and thank you Kropf Incorporated has provided the following data concerning one of the products in its standard cost system. Variable manufacturing overhead is applied to...
-
Estimate K at 100 C for the reaction 2 SO 2 (g) + O 2 (g) 2 SO 3 (g). Use data from Table 13.8 and Figure 13-10. Table 13.8 Figure 13-10 TABLE 13.8 Equilibrium Constants, K, for the Reaction 2 SO(g)...
-
To establish the law of conservation of mass, Lavoisier carefully studied the decomposition of mercury(II) oxide: (a) Show that the partial pressure of O 2 (g) in equilibrium with HgO(s) and Hg(l) at...
-
Slackur Company is a very profitable small business. However, it has not given much consideration to internal controls. For example, in an attempt to keep its clerical and office expenses to a...
-
what extent do elite networks shape policy and governance, and how transparent are these networks to public scrutiny ? Explain
-
What strategies do you employ to mediate conflicts within a team to ensure that disagreements are resolved constructively and synergistically?
-
What goal(s) do you think the communication was intended to achieve? What type of promotional communication is it and why? What do you believe to be the advertising theme or central idea of the...
-
What Do You Know About Amazon Associate Program? What Would You Do To Increase Your Earnings With Amazon Associate Program? Is Affiliate Marketing And Referral Marketing One And The Same? What is...
-
As a leader, what do you think are important elements of a leadership team made up of those senior people that you will surround yourself with? Do you have (or have you had) a mentor? If so, how have...
-
A 480/2,400-V rms step-up ideal transformer delivers 50 kW to a resistive load. Calculate: (a) The turns ratio (b) The primary current (c) The secondary current
-
Prove the result that the R 2 associated with a restricted least squares estimator is never larger than that associated with the unrestricted least squares estimator. Conclude that imposing...
-
Absorption costing and production-volume variance-alternative capacity bases. Earth Light First (ELF), a producer of energy-efficient light bulbs, expects that demand will increase markedly over the...
-
Operating income effects of denominator-level choice and disposal of production-volume variance 1. If ELF sells all 220,000 bulbs produced, what would be the effect on operating income of using each...
-
Cost allocation downward demand spiral Deli One operates a chain of 10 hospitals in the Los Angeles area. Its central food-catering facility, Deliman, prepares and delivers meals to the hospitals. It...
-
By the help of Conditioning Formatting (not manually!) underline the managers who impacted with the highest and the lowest sales. Post your results in Problem1 sheet
-
Galloway Inc is an over levered public company, with two classes of shares. Since the insiders (who run the company) own the voting shares, the company has the luxury of decreasing its debt ratio...
-
Zheng Corporation plans to issue new bonds to finance its expansion plans. In its efforts to price the issue, Zheng Corporation has identified a company of similar risk with an outstanding bond issue...

Study smarter with the SolutionInn App


