Treating benzene with fuming sulfuric acid yields benzenesulfonic acid, which is formed by the following reaction: The
Question:
Treating benzene with fuming sulfuric acid yields benzenesulfonic acid, which is formed by the following reaction:
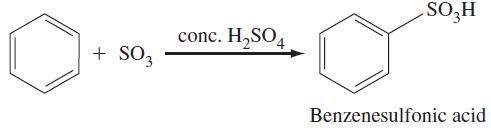
The SO3 that participates in the reaction above is formed by the reaction of sulfuric acid molecules:
![]()
The reaction of benzene and SO3 to give benzenesulfonic acid involves the following elementary processes. SO3 acts as an electrophile in a reaction with benzene, forming an arenium ion. A proton is then transferred from the arenium ion to HSO4–, forming the benzenesulfonate ion, C6H5SO3–, and H2SO4. Finally, C6H5SO3– is protonated by H3O+ to give benzenesulfonic acid and a water molecule. Write balanced chemical equations for these elementary processes, using curved arrows to show the movement of electrons.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





