Use data from Table 7.2, together with the fact that r H = -3509 kJ mol
Question:
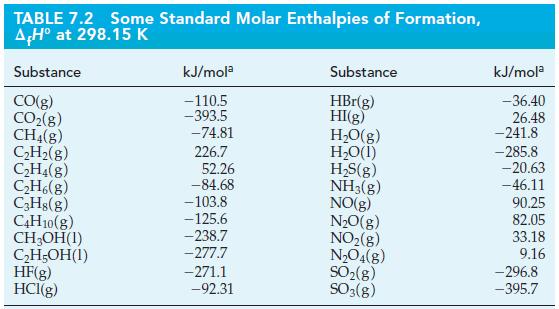
Use data from Table 7.2, together with the fact that ΔrH° = -3509 kJ mol-1 for the complete combustion of pentane, C5H12(l), to calculate ΔrH° for the reaction below.
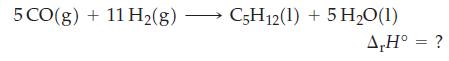
Table 7.2
Transcribed Image Text:
5 CO(g) + 11 H₂(g) - C5H12(1) + 5 H₂O(1) A,H° = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To determine the standard enthalpy change AH for the ...View the full answer

Answered By

Sheikh Muhammad Ibrahim
During the course of my study, I have worked as a private tutor. I have taught Maths and Physics to O'Level and A'Level students, as well as I have also taught basic engineering courses to my juniors in the university. Engineering intrigues me alot because it a world full of ideas. I have passionately taught students and this made me learn alot. Teaching algebra and basic calculus, from the very basics of it made me very patient. Therefore, I know many tricks to make your work easier for you. I believe that every student has a potential to work himself. I am just here to polish your skills. I am a bright student in my university. My juniors are always happy from me because I help in their assignments and they are never late.
4.90+
14+ Reviews
24+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use data from Table to estimate ÎH for the combustion of methane (CH4), as shown below: Table s | 14 39 95 45 72 1 1419 6847064968 77386 42222 34 985 0302 121 Si H C O 437 490 9 31222241122...
-
Create an accompanying report that draws on relevant theories and concepts within Block 1 to explain and justify the decisions made in producing your team's piece of marketing communication. Context...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The symmetrical three-phase load is A-connected, with Z-(4+ j3) 22 and the phase voltage 220 V, find IL, IP, UL and P of the three loads. 7.(10 score) The symmetrical three-phase load is Y-connected....
-
Assume that you must make a presentation to a client explaining the difference between prime and conversion costs. The client makes and sells 200,000 cookies per week. The client tells you that her...
-
Given the following data, compute the historical volatility of the constant maturity forward rates. Weekly Forward Price Data Constant Maturity Simple Forward Rate i(n,s) S 1.00 0.1230 0.1260 0.1270...
-
NPV PROFILES: TIMING DIFFERENCES An oil-drilling company must choose between two mutually exclusive extraction projects, and each costs $12 million. Under Plan A, all the oil would be extracted in 1...
-
Universal Corporation is planning to invest in a security that has several possible rates of return. Given the following probability distribution of returns, what is the expected rate of return on...
-
Help Save & Exit Kerekes Manufacturing Corporation has prepared the following overhead budget for next month. Activity level Variable overhead costs: Supplies Indirect labor Fixed overhead costs:...
-
Read the case San Lorenzo Trad., Inc. v. Hernndez, Gonzlez, Figueroa & Merly, 114 DPR 704 (1983), indicated in the required resources of module 3. Once you have read the case, answer the following...
-
Use data from Table 7.2 and r H for the following reaction to determine the standard enthalpy of formation of CCl 4 (g) at 25 C and 1 bar. Table 7.2 CH4(g) + 4Cl(g) CCl4(g) + 4HCI(g) AH -397.3 kJ mol
-
Use data from Table 7.2 to determine the standard heat of combustion of C 2 H 5 OH(l), if reactants and products are maintained at 25 C and 1 bar. Table 7.2 TABLE 7.2 AH at 298.15 K Substance CO(g)...
-
Presented below are three independent situations. (a) Snider Corporation incurred the following costs in connection with the issuance of bonds: (1) Printing and engraving costs $40,000; (2) Legal...
-
You have just been hired as a financial analyst for Lydex Company, a manufacturer of safety helmets. Your boss has asked you to perform a comprehensive analysis of the company s financial statements,...
-
For our first discussion you should locate a research article in which a quantitative study is reported. This article should not be a theoretical article or a methods article, but should describe...
-
A box is separated by a partition which divides its volume in the ration of 3:1. the larger portion of the box contains 1000 molecules of Ne gas; the smalled portion contains 100 molecules of He gas....
-
The process of translating an idea into goods and services that create value or for which clients will pay is called
-
Let f be twice differentiable with f(0) = 6, f(1) = 8, and f'(1) = 7. Evaluate the following integral. [ = 0 0 xf" (x)dx
-
Why is the rigidity of the machine tool an important consideration in the selection of the cutting tool material?
-
Proposals have been made to ?sail? spacecraft to the outer solar system using the pressure of sunlight, or even to propel interstellar spacecraft with high-powered, Earth-based lasers. Sailing...
-
Classification of Costs and Interest Capitalization On January 1, 2010, Blair Corporation purchased for $500,000 a tract of land (site number 101) with a building. Blair paid a real estate brokers...
-
Interest during Construction Grieg Landscaping began construction of a new plant on December 1, 2010. On this date the company purchased a parcel of land for $139,000 in cash. In addition, it paid...
-
Capitalization of Interest Laserwords Inc. is a book distributor that had been operating in its original facility since 1985. The increase in certification programs and continuing education...
-
Napa Tours Co. is a travel agency. The nine transactions recorded by Napa Tours during April 2018, its first month of operations, are indicated in the following T accounts: Cash Equipment Dividends...
-
please help The following data from the just completed year are taken from the accounting records of Mason Company Sales Direct lobor cost Raw material purchases Selling expenses Administrative...
-
It is February 1 5 , 2 0 2 4 , and you are visiting with your friends Carl and Ellie Fredricksen at their Calgary home. Carl is 6 3 years old and Ellie is 6 5 years old. Carl: We have big news. Ellie...

Study smarter with the SolutionInn App


