Use data from Table 7.2 to determine the standard heat of combustion of C 2 H 5
Question:
Use data from Table 7.2 to determine the standard heat of combustion of C2H5OH(l), if reactants and products are maintained at 25 °C and 1 bar.
Table 7.2
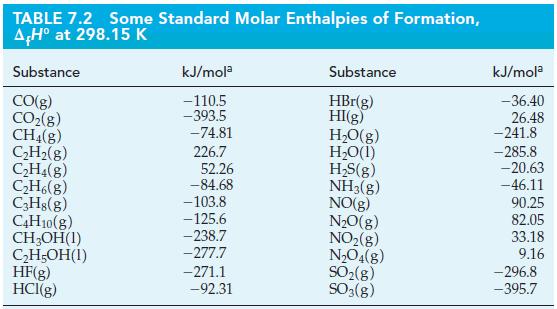
Transcribed Image Text:
TABLE 7.2 AH° at 298.15 K Substance CO(g) CO₂(g) CH₂(g) C₂H₂(g) C₂H4(g) C₂H6(g) C3H8(g) C4H10(g) CH3OH(1) C₂H5OH(1) Some Standard Molar Enthalpies of Formation, HF(g) HCI(g) kJ/mola -110.5 -393.5 -74.81 226.7 52.26 -84.68 -103.8 -125.6 -238.7 -277.7 -271.1 -92.31 Substance HBr(g) HI(g) H₂O(g) H₂O(1) H₂S(g) NH3(g) NO(g) N₂O(g) NO₂(g) N₂O4(g) SO₂(g) SO3(g) kJ/mola -36.40 26.48 -241.8 -285.8 -20.63 -46.11 90.25 82.05 33.18 9.16 -296.8 -395.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Balanced chemical equation for the combustion ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The standard heat of combustion of liquid n-octane to form CO 2 and liquid water at 25C and 1 atm is H c = 5471 kJ/mol. (a) Briefly explain what that means. Your explanation may take the form When...
-
The standard heat of the combustion reaction of liquid n-hexane to form CO 2 (g) and H 2 O(l), with all reactants and products at 77F and 1 atm, is H r = 1:791 10 6 Btu. The heat of vaporization of...
-
In Problem 3.67 on page 135, you were introduced to a tea-bag-filling operation. An important quality characteristic of interest for this process is the weight of the tea in the individual bags. The...
-
Listed here are the total costs associated with the production of 10,000 Blu-ray Discs (BDs) manufactured by New Age. The BDs sell for $15 each. Required 1. Classify each cost and its amount as (a)...
-
Suppose you want to change your budget to increase your savings. What could you do?
-
CHOOSING MANDATORY PROJECTS ON THE BASIS OF LEAST COST Kim Inc. must install a new air conditioning unit in its main plant. Kim must install one or the other of the units; otherwise, the highly...
-
a. How would you describe Wallys present investment program? How do you think it fits him and his investment objectives? b. Consider the Hydro-Electric stock. 1. Determine the amount of annual...
-
On January 1, 2022, the ledger of Sunland Company contained these liability accounts. Accounts Payable $44,400 Sales Taxes Payable 8,500 Unearned Service Revenue 20,900 During January, the following...
-
Simplex Corporation provides the following information at the end of 2015. Salaries payable to workers at the end of the year ................ $ 3,500 Advertising expense for the year...
-
Use data from Table 7.2, together with the fact that r H = -3509 kJ mol -1 for the complete combustion of pentane, C 5 H 12 (l), to calculate r H for the reaction below. Table 7.2 5 CO(g) + 11 H(g)...
-
Use data from Appendix D to calculate r H for the following reaction at 25 C. Fe2O3(s) + 3 CO(g) 2 Fe(s) + 3 CO(g) A,H = ?
-
What is the amount of the credit to Social Security tax payable-OASDI?
-
Although the Chen Company's milling machine is old, it is still in relatively good working order and would last for another 10 years. It is inefficient compared to modern standards, though, and so...
-
PART-3: OFFLINE QUESTIONS - Upload files using the submission link. 1. In 2020 Starbucks began a secret project to develop a competing product against the Keurig Single Serve coffee brewer. The...
-
As a leader, what are your highest values? o What's the contribution you want to make as a leader o What makes you distinct as a leader? o Drawing from StrengthsFinder 2.0 what are your strengths as...
-
The transmitted energy expands out into space as it propagates at 3 GHz between the transmitter and the receiver over 30 km distance. Calculate the free space loss using a suitable formula and any...
-
What is the company featured in this episode of Undercover Boss? List 3 good professional activities that the CEO/president learned about their company by going undercover? List areas of the...
-
Over the years, tool materials have been developed which have allowed significant increases in MRR. Nevertheless, HSS is still widely used. Under what conditions might HSS be the material of choice?
-
A spacecraft has left the earth and is moving toward Mars. An observer on the earth finds that, relative to measurements made when the spacecraft was at rest, its a. length is shorter b. KE is less...
-
Nonmonetary Exchanges Holyfield Corporation wishes to exchange a machine used in its operations. Holyfield has received the following offers from other companies in the industry. 1. Dorsett Company...
-
Nonmonetary Exchanges on August 1, Hyde, Inc. exchanged productive assets with Wiggins, Inc. Hydes asset are referred to below as Asset A, and Wiggins is referred to as Asset B. The following facts...
-
Nonmonetary Exchanges During the current year, Marshall Construction trades an old crane that has a book value of $90,000 (original cost $140,000 less accumulated depreciation $50,000) for a new...
-
Michael will earn $148,000 in 2020. a. How much in total will be paid into the entire OASDI system (including Medicare) on behalf of this income? ___________________________ b. How much will Michael...
-
Cost = 125 000 Resdiual Value = 5000 Useful life = 10 years .. using both stright line and double declining method determine 1. Accumlated depreciation at the end of year 2 2. Book value at the...
-
TB Problem Qu. 9-373 Varriano Corporation bases its budgets on... 2 Variano Corporation bases its budgets an the activity measure customers served. During October, the company planned to serve 49,000...

Study smarter with the SolutionInn App


