Use data from Appendix D to calculate r H for the following reaction at 25 C.
Question:
Use data from Appendix D to calculate ΔrH° for the following reaction at 25 °C.
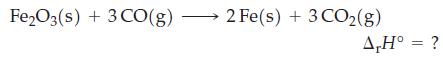
Transcribed Image Text:
Fe2O3(s) + 3 CO(g) 2 Fe(s) + 3 CO₂(g) A,H° = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
To calculate rH for the reaction at 25 C we need to use the enthalpy of f...View the full answer

Answered By

Albert Kinara
i am an expert research writer having worked with various online platform for a long time. i also work as a lecturer in business in several universities and college part time and assure you well researched and articulate papers. i have written excellent academic papers for over 5 year and have an almost similar experience experting many clients in different units. bachelor of commerce (finance)
masters in strategic management
phd finance
4.60+
26+ Reviews
48+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use data from Appendix C to calculate the equilibrium constant, K, at 298 K for each of the following reactions: H2(g) + 12(g) 2 HI(g) C2H5OH (g)- C2H4(g) + H2O(g)
-
The decomposition of limestone, CaCO 3 (s), into quicklime, CaO(s), and CO 2 (g) is carried out in a gas-fired kiln. Use data from Appendix D to determine how much heat is required to decompose 1.35...
-
Calculate the equilibrium constant K for the following reaction at 25C from standard electrode potentials. Fe3 + (aq) + Cu(s) Fe2+(aq) + Cu2+(aq) The equation is not balanced.
-
A trader has a soft limit of $8MM USD and a hard limit of $10MM USD. Their current NOP is $3MM USD. They purchase $6MM USD, sell $1MM USD, and then purchase another $5MM USD. Which of the following...
-
Eastman-Kodak manufactures digital cameras and must compete on lean manufacturing concepts. Match each of the following activities that it engages in with the lean manufacturing concept it strives to...
-
What is a non-refundable tax credit? What is a refundable tax credit? What is the difference between a tax deduction and a tax credit? Which is more valuable?
-
MIRR A firm is considering two mutually exclusive projects, X and Y, with the following cash flows: 0 2 $700 $50 $300 $100 $100 $1,000 2$1,000 2$1,000 Project X Project Y 3 4 $400 $50 1 The projects...
-
The Walt Disney Company has four profitable business segments, described as follows: Media Networks: The ABC television and radio network, Disney channel, ESPN, A& E, E!, and Disney.com. Parks and...
-
a. what is Cascadia's level of gross investment? b. What is the expenditure-based estimate of Cascadia's GDP? c. What is the income-based estmate of GDP? d. What is Cascadia's GDP? e. If Cascadia's...
-
Calculate the enthalpy and entropy of saturated isobutane vapor at 360 K from the following information: 1. Table 6.1 gives compressibility-factor data (values of Z) for isobutane vapor. 2. The vapor...
-
Use data from Table 7.2 to determine the standard heat of combustion of C 2 H 5 OH(l), if reactants and products are maintained at 25 C and 1 bar. Table 7.2 TABLE 7.2 AH at 298.15 K Substance CO(g)...
-
Use standard enthalpies of formation from Table 7.2 to determine r H at 25 C for the following reaction. Table 7.2 2 Cl(g) + 2 HO(1) 4 HCl(g) + O(g) A,H = ?
-
Listed below are several terms and phrases associated with the accounting processing cycle. Pair each item from List A (by letter) with the item from List B that is most appropriately associated with...
-
Assume there is a national lottery in the winning ticket is worth $10 million one winning ticket will be selected if there are 225 million tickets sold. What is the chance that a buyer of one ticket...
-
Description: Reference: Basu Thakur. (2015). PostcolonialTheory and Avatar (pp. 85-150,157-172). Bloomsbury PublishingUSAPre-Peer Paper Review for the Postcolonial Application Paper 1: Collecting...
-
NOT ASKING THE ACTUAL SHEAR STRESS. Please READ! Derive the shear stress distributed equation over the cross-section. Derive the equation and plot. 15 15 30 15 15 120 -90 20 0.5 m 72 kN 20 20 40 40...
-
You are the cost accountant of an engineering concern which has three departments - preparation, machining and assembly. The budgeted direct labour hours for the workshops are 8,000, 12,000 and...
-
What alternative to fostering fun and enjoyment at work do you think might have worked for Zappos?
-
Why is there no universal cutting tool material?
-
Modify the counter from Exercise 5.44 such that the counter will either increment by 4 or load a new 32-bit value, D, on each clock edge, depending on a control signal Load. When Load = 1, the...
-
Purchases by Deferred Payment, Lump-Sum, and Nonmonetary Exchanges Klamath Company, a manufacturer of ballet shoes, is experiencing a period of sustained growth. In an effort to expand its production...
-
Acquisition, Improvements, and Sale of Realty Tonkawa Company purchased land for use as its corporate headquarters. A small factory that was on the land when it was purchased was torn down before...
-
Accounting for Self-Constructed Assets Troopers Medical Labs, Inc., began operations 5 years ago producing stetrics, a new type of instrument it hoped to sell to doctors, dentists, and hospitals. The...
-
IFRS is comprised of: a.International Financial Reporting Standards and FASB financial reporting standards. b.International Financial Reporting Standards, International Accounting Standards, and...
-
Enter the missing dollar amounts for the income statement for each of the following independent cases
-
MULTIPLE CHOICE ASAP PLS WILL UPVOTE 1 Multiple Choice $106,200. $13,200. $199,200. $267,000. $246,000.

Study smarter with the SolutionInn App


