Use standard enthalpies of formation from Table 7.2 to determine r H at 25 C for
Question:
Use standard enthalpies of formation from Table 7.2 to determine ΔrH° at 25 °C for the following reaction.
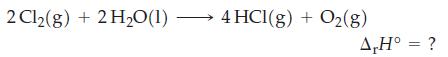
Table 7.2
Transcribed Image Text:
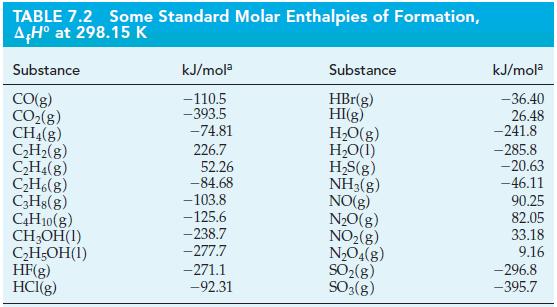
2 Cl₂(g) + 2 H₂O(1) 4 HCl(g) + O₂(g) A,H° = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Balanced chemical reaction for Cl and water 2Cl 2HO0 4HC1...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use standard enthalpies of formation from Table 7.2 and equation (7.22) to determine the standard enthalpy of reaction in the following reactions. Table 7.2 Eq. 7.22 (a) C3H8(g) + H(g) CH6(g) +...
-
The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ/mol, respectively. Using these data and the thermodynamic data in Appendix C, calculate the overall enthalpy change for each...
-
Use data from Table 7.2 and r H for the following reaction to determine the standard enthalpy of formation of hexane, C 6 H 14 (l), at 25 C and 1 bar. Table 7.2 2 C6H14(1) + 19 O2(g) 12 CO(g) + 14...
-
Evaluate the cube root of = 27cis (240). Then raise them to the cube. Show the steps of your reasoning.
-
This chapter described the purpose of managerial accounting in the context of the current business environment. Review the home electronics section of your local newspaper; the Sunday paper is often...
-
Linda neglected to complete herT1 General in time for the filing deadline of April 30, 2014. This would not be a problem if she did not owe any tax. However, after completing her tax return, she...
-
IRR AND NPV A company is analyzing two mutually exclusive projects, S and L, with the following cash flows: 0 2 $10 $800 $250 $250 $900 $0 2$1,000 2$1,000 Project S Project L 3 4 $10 $400 1 The...
-
Douglas Company's beginning inventory and purchases during the fiscal year ended December 31, 20--, were as follows: There are 1,000 units of inventory on hand on December 31. REQUIRED 1. Calculate...
-
Bond Valuation. Mia wants to invest in Treasury bonds that have a par value of $20,000 and a coupon rate of 6.3%. The bonds have a 14-year maturity, and Mia requires a 7% return How much should Mia...
-
Alabama Atlantic is a lumber company that has three sources of wood and five markets to be supplied. The annual availability of wood at sources 1, 2, and 3 is 15, 20, and 15 million board feet,...
-
Use data from Appendix D to calculate r H for the following reaction at 25 C. Fe2O3(s) + 3 CO(g) 2 Fe(s) + 3 CO(g) A,H = ?
-
Use the data in Figure 7-18 and information to establish possible relationships between the molecular structure of the hydrocarbons and their standard enthalpies of formation. Figure 7-18 Positive...
-
Use the process described in Exercises 8 and 9 to plan and role-play another situation. Imagine that a coworkers behavior falls into the obnoxious aggression category of radical candor. Earlier in...
-
Using the techniques of dimensional analysis, and assuming that experimentation shows the dimensionless number to be 1, derive the following equation: E v = Job card two The results of an ultrasonic...
-
Given the historical cost of product Carla Vista is $13, the selling price of product Carla Vista is $15, costs to sell product Carla Vista are $3, the replacement cost for product Carla Vista is...
-
What causes of outliers in statistics and when I create a boxplot why do I not see the outliers. What steps are to take in creating a boxplot?
-
A year-end cut-off error occurred in 2017. A large shipment of nonperishable supplies arrived from South America on the last day of 2017 and had been left in the shipping containers outside the main...
-
15. [5] It's not so difficult to incorporate time-varying volatility into the BSM model as long as the time variation is not random. Assume a BSM economy, but this time, assume that the volatility of...
-
Why is the PVD process used to coat HSS tools?
-
What are the six activities involved in the physical supply/distribution system?
-
Capitalization of Interest Langer Airline is converting from piston-type planes to jets. Delivery time for the jets is 3 years, during which substantial progress payments must be made. The...
-
Capitalization of Interest Vania Magazine Company started construction of a warehouse building for its own use at an estimated cost of $5,000,000 on January 1, 2009, and completed the building on...
-
Nonmonetary Exchanges You has two clients that are considering trading machinery with each other. Although the machines are different from each other, you believe that an assessment of expected cash...
-
I am struggling to find the answers to all of the questions! please help! :) E3-8 Determining Accounting Equation Effects and Net Income [LO 3-2, LO 3-3) The following transactions occurred during a...
-
What is the expected pay-off in a Colonel Blotto game where Blotto has troops in the formation of 3100 and the enemy has troops in the formation of 2100?
-
12. At the beginning of the period, the Assembly Department budgeted direct labor of $123,500 and property tax of $14,700 for 6,500 hours of production. The department actually completed 7,200 hours...

Study smarter with the SolutionInn App


