Use the data in Figure 7-18 and information to establish possible relationships between the molecular structure of
Question:
Use the data in Figure 7-18 and information to establish possible relationships between the molecular structure of the hydrocarbons and their standard enthalpies of formation.
Figure 7-18
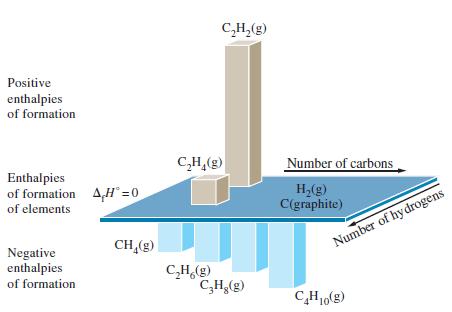
Transcribed Image Text:
Positive enthalpies of formation Enthalpies of formation AHⓇ=0 of elements Negative enthalpies of formation CH₂(g) C₂H₂(g) C₂H₂(g) Number of carbons H₂(g) C(graphite) J C₂H.(g) C₂H₂(g) Number of hydrogens C₂H₁0(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Polycyclic aromatic hydrocarbons (PAHs)-formed during the incomplete burning of oil, gas, or coal-are considered to be potential dangerous pollutants by the Environmental Protection Agency (EPA)....
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Ants release formic acid (HCOOH) when they bite. Use the data in Table 7.2 and the standard enthalpy of combustion for formic acid ( r H = -255 kJ/mol) to calculate the standard enthalpy of formation...
-
The owner of a moving company typically has his most experienced manager predict the total number of labor hours that will be required to complete an upcoming move. This approach has proved useful in...
-
Mission Oak Company produces oak bookcases to customer order. It received an order from a customer to produce 5,000 bookcases. The following information is available for the production of the...
-
Akida has invested $10,000 in an 18-month GIC that pays 6.25 percent, compounded annually. How much interest will Akida receive at maturity?
-
CAPITAL BUDGETING CRITERIA: MUTUALLY EXCLUSIVE PROJECTS Project S costs $15,000, and its expected cash flows would be $4,500 per year for 5 years.Mutually exclusive Project L costs $37,500, and its...
-
Revenue usually is recognized at the point of sale. Under special circumstances, however, bases other than the point of sale are used for the timing of revenue recognition. Required 1. Why is the...
-
Stock A standard deviation = 21% Stock B standard deviation = 10% In a world where investors are risk averse and can hold only one stock, we can conclude that the required rate of return on Stock A...
-
For each of the following scenarios, indicate whether or not independence-related SEC rules are being violated, assuming that the audit entity is a public company. Briefly explain why or why not. a....
-
Use standard enthalpies of formation from Table 7.2 to determine r H at 25 C for the following reaction. Table 7.2 2 Cl(g) + 2 HO(1) 4 HCl(g) + O(g) A,H = ?
-
Use the information given here, data from Appendix D, and equation (7.22) to calculate the standard enthalpy of formation per mole of ZnS(s). Eq.7.22 2 ZnS(s) + 3O(g) 2 ZnO(s) + 2 SO(g) A,H -878.2 kJ...
-
Show in two dimensions that the maximum shear stress m = {[(xx yy)/2]2 + (xy)2}(1/2) is invariant under a rotation of the reference axes and is equal to one-half the difference of the major and minor...
-
3.6. Explain and discuss the potential benefits to be gained by using blade twist, plan- form taper, low solidity, large radius, and low rotational speed for the main rotor of a heavy lift helicopter...
-
2. A VRM (Voltage Regulator Modul) is used to supply the voltageto the CPU of a computer. In the new generation of microprocessors,whose power consumption is 100W, the input voltage to the VRM is12V...
-
Alvarado Company produced 6,400 units of product that required 5.5 standard direct labor hours per unit. The standard variable overhead cost per unit is $5.80 per direct labor hour. The actual...
-
A company must decide between scrapping or reworking units that do not pass inspection. The company has 16,000 defective units that have already cost $132,000 to manufacture. The units can be sold as...
-
according to the phase rule, the triple point of a pure substance is A. invariant B. u nivariant C. bivariant D. none of the above
-
What is impact strength and how is it measured?'
-
Catalytic hydrogenation of naphthalene over PdC results in rapid addition of 2 moles of H 2 . Propose a structure for this product.
-
Costs of Acquisition the invoice price of a machine is $50,000. Various other costs relating to the acquisition and installation of the machine including transportation, electrical wiring, and...
-
Cost of Land vs. BuildingEthics Tones Company purchased a warehouse in a downtown district where land values are rapidly increasing. Gerald Carter, controller, and Wilma Ankara, financial vice...
-
In recent years, the Wall Street Journal has indicated that many companies have changed their accounting principles. What are the major reasons why companies change accounting methods?
-
Sumitra devi, an Indian citizen, has a property in Bangladesh and is a resident. She also has property in India where she earns 500,OOO rupees ( 1 rupee =2 BDT)? What is her foreign income?
-
4. Write an answer to the following question: What circumstances would cause the Allowance for doubtful Accounts to have a debit balance prior to adjustment?
-
Is there any specific accounting regulations and requirements for SMEs in Oman?

Study smarter with the SolutionInn App


