Use the following data together with other data from the text to determine the temperature at which
Question:
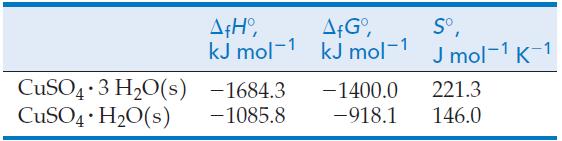
Use the following data together with other data from the text to determine the temperature at which the equilibrium pressure of water vapor above the two solids in the following reaction is 75 Torr.
![]()
Transcribed Image Text:
CuSO4 3 H₂O(s) — CuSO4 · H₂O(s) + 2 H₂0(g) . .
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
To determine the temperature at which the equilibrium pressure of water vapor above the two solids in the given reaction is 75 Torr we can use the Van...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Suppose that you have $250 to invest. a). You decide to store the $250 in cash under your mattress. If inflation is 3 percent, what will the real value of that cash be after one year under the...
-
In a study of the reaction 3Fe(s) + 4H2O(g) Fe3O4(s) + 4H2(g) at 1200 K, it was observed that when the equilibrium partial pressure of water vapor is 15.0 torr, the total pressure at equilibrium is...
-
Four vapor pressure data pointstwo representing solidvapor equilibrium and two representing liquidvapor equilibriumare available for a compound: A. Give your best estimate of the triple point...
-
Suppose you needed a material that could absorb heat without having its temperature increase very much. Would you choose aluminum or water? Make sure your explanation uses the concept of specific...
-
In Paige Company, direct labor is $20 per hour. The company expects to operate at 10,000 direct labor hours each month. In January 2014, direct labor totaling $204,000 is incurred in working 10,400...
-
How many moles of sugar (sucrose) are there in 5 L of sugar water that has a concentration of 0.5 M? (a) 5.5 moles (b) 5.0 moles (c) 2.5 moles (d) 1.5 moles
-
What is the key feature of activity-based responsibility accounting?
-
Collier Manufacturing Co. operates on a modified wage plan. During one weeks operation, the following direct labor costs were incurred: The employees are machine operators. Piece rates vary with the...
-
[The following information applies to the questions displayed below] Ramirez Company installs a computerized manufacturing machine in its factory at the beginning of the year at a cost of $86,200....
-
From the data given in Exercise 72, estimate a value of r S at 298 K for the reaction Exercise 72 Sodium carbonate, an important chemical used in the production of glass, is made from sodium...
-
The term thermodynamic stability refers to the sign of r G . If r G is negative, the compound is stable with respect to decomposition into its elements. Use the data in Appendix D to determine...
-
Two fair dice are rolled one after the other. Construct a sample space and determine the probability that the sum of the dots on the dice total. 3.
-
What is among the most important things you should do in a negotiation? What is among the most important things you should do in a negotiation? Try to get your way on as many issues as possible. Find...
-
analyze the following column values and answer question: Value Value Label Frequency Percentage Weighted Percentage 1 - 87 Number of children Notes: _ _ = Number of children 113,819 25.78 36.41 88...
-
Reflect on the following questions. Post your response to the discussion board. Post your discussion post by Thursday . 1 peer response due by Sunday. This discussion has two parts: 1) Mediators and...
-
Give your overall opinion . What do you think about neuromarketing? Is it usefull or is it a waste of time? Some people think this practice is "Orwellian", do you agree? Can marketers manage the...
-
Question 1. Let z= f(x,y), x = g (s, t). and ' y = h (s, t). with f, g & h all differentiable. (a) Set up an appropriate tree diagram for the of chain rule as done in this module's Use video lessons:...
-
Determine the average power absorbed by each resistor in the circuit of Fig. 13.123. 20 20 @ 100 80cos 41
-
What are the key elements of a system investigation report?
-
Price and efficiency variances, journal entries. The Monroe Corporation manufactures lamps. It has set up the following standards per finished unit for direct materials and direct manufacturing...
-
Continuous improvement the Monroe Corporation sets monthly standard costs using a continuous-improvement approach. In January 2009, the standard direct material cost is $45 par unit and the standard...
-
Materials and manufacturing labor variances, standard costs. Dunn, Inc. is a privately held furniture manufacturer. For August 2009, Dunn had the following standards for one of its products, a wicker...
-
Fill out a spreadsheet using the following information to perform a NPV analysis. Revenues in each of years 1-3 = $20,000 Year 0 initial investment = $40,000 Inventory level = $10,000 in year 1,...
-
A firm has $9.5 Billion debt outstanding, with a yield to maturity of 5.3% and a coupon rate of 4.6%. They have 148 million preferred shares outstanding, currently trading at $92.29. They also have...
-
PART II (35 possible marks) Woolsworth Corporation manufactures and sells woolen jackets. It is ready to begin its fourth quarter, in which they have their highest sales. The company has approached...

Study smarter with the SolutionInn App


