What response would you expect in the apparatus of Figure 5-4 if the solution tested were 1.0
Question:
What response would you expect in the apparatus of Figure 5-4 if the solution tested were 1.0 M HCl? What response would you expect if the solution were both 1.0 M HCl and 1.0 M CH3COOH?
Figure 5-4
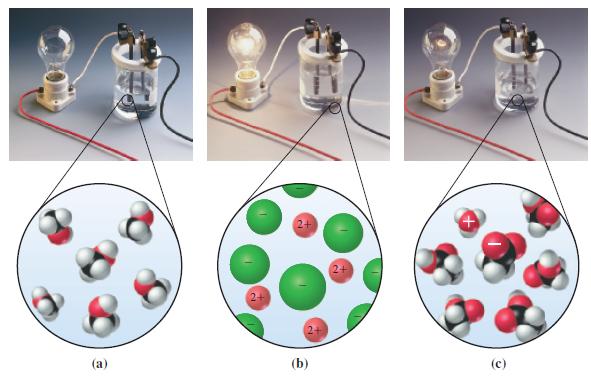
Transcribed Image Text:
(a) 2+ (b) 2+ + (c)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Since HCl solution is a strong electrolyte It is comp...View the full answer

Answered By

Douglas Makokha
Unlock Academic Success with Dedicated Tutoring and Expert Writing Support!
Are you ready to excel in your academics? Look no further! As a passionate tutor, I believe that dedication and hard work are the keys to achieving outstanding results. When it comes to academics, I strive to provide nothing but the best for every student I encounter.
With a relentless thirst for knowledge, I have extensively researched numerous subjects and topics, equipping myself with a treasure trove of answers to tackle any question that comes my way. With four years of invaluable experience, I have mastered the art of unraveling even the most intricate problems. Collaborating with esteemed writers has granted me exclusive access to the trade secrets utilized by the industry's top professionals.
Allow me the pleasure of assisting you with your writing assignments. I thrive on challenges and will guide you through any obstacles you may face. Together, we will unlock your academic potential and pave the way for your success.
4.90+
62+ Reviews
349+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The fluctuating price of oil has a big impact on the cost of a supply chain. As oil prices increase, so does the cost of transportation. Recent fluctuations in the price of oil have taught supply...
-
What differences, if any, would you expect in the properties of castings made by permanent-mold vs sand-casting methods?
-
You are considering three stocksA, B, and Cfor possible inclusion in your investment portfolio. Stock A has a beta of 0.80, stock B has a beta of 1.40, and stock C has a beta of 0.30. a. Rank these...
-
Let a 0. Solve |x| = 3.
-
Assume all the information is the same as in Custom Freight Systems (A), but instead of receiving one outside bid, Logistics receives two. The new bid is from World Services for $195 per hundred...
-
Search the Internet for some time series data that relates to sustainability, for example, environmental emissions. What types of patterns do these data exhibit? Apply forecasting techniques in this...
-
30. What are reasons why companies provide nonqualified deferred compensation plans for certain employees?
-
The following partially completed process cost summary describes the May production activities of Raman Company. Its production output is sent to its warehouse for shipping. Prepare its process cost...
-
H a e Heading 1 Heading 2 Title Subtitle Subtle Paragraph Styles Steve Harrington of the controller's office of Scoops Ahoy, Inc. was given the assignment of recording several transactions and...
-
You are given the four solids, K 2 CO 3 , CaO, ZnSO 4 , and BaCO 3 , and three solvents, H 2 O(l), HCl(aq), and H 2 SO 4 (aq). You are asked to prepare four solutions, each containing one of the four...
-
(A) Write a net ionic equation to represent the reaction of aqueous ammonia with propionic acid, CH 3 CH 2 COOH. Assume that the neutralization reaction goes to completion. What is the formula of the...
-
1. As a manager at Lululemon, what management functions would repair the damage that has been done to the brand, and help the company to move forward? 2. How was Chip Wilsons management so successful...
-
Economic order quantity; order cost; carrying cost Starr Company predicts that it will use 360,000 units of material during the year. The material is expected to cost $5 per unit. Starr anticipates...
-
Requirement 1. Prepare a horizontal analysis of the comparative income statement of McCormick Designs, Inc. Round percentage changes to one decimal place. (Round the percentages to one decimal place,...
-
GAAP looks to compare entities with an apples-to-apples valuation so that the entities can be viewed by interested parties to compare financial values and how effectively and efficiently they use...
-
Prepare a statement of cash flows for Wu using the indirect method.
-
A plate of steel with a central through-thickness flaw of length 16 mm is subjected to a stress of 350 MPa normal to the crack plane. If the yield strength of the material is 1400 MPa what is the...
-
(a) Show that if ki and fi are eigenvalues and eigenfunctions of the linear operator A, then cki and fi are eigenvalues and eigenfunctions of cA. (b) Give an operator whose eigenvalues are 1/2, 3/2,...
-
Vince, Inc. has developed and patented a new laser disc reading device that will be marketed internationally. Which of the following factors should Vince consider in pricing the device? I. Quality of...
-
In what ways are the inventory accounts of a retailing company different from those of a manufacturing company?
-
Why inventories should be included in (a) A statement of financial position and (b) The computation of net income?
-
What is the difference between a perpetual inventory and a physical inventory? If a company maintains a perpetual inventory, should its physical inventory at any date be equal to the amount indicated...
-
Stockholders Equity Transactions, Journal Entries, and T-Accounts The stockholders equity of Fremantle Corporation at January 1 follows: 8 Percent preferred stock, $110 par value, 20,000 shares...
-
How does tax evasion differ from tax avoidance?
-
Question 14 (1 point) Android is used as a non-mobile operating system. True False

Study smarter with the SolutionInn App


