Write an acceptable value for each of the missing quantum numbers. (a) n = 3, (b) n
Question:
Write an acceptable value for each of the missing quantum numbers.
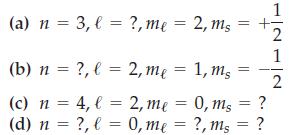
Transcribed Image Text:
(a) n = 3, (b) n (c) n = 4, l = (d) n = ?, l= ?, l = ?, me = 2, ms 2, me = 1, ms 2, me 0, me = = 0, ms ?, ms ? = ? 1 1 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Acceptable values for the missing quantum numbers a n 3 l 2 ml 2 ms 12 b n 4 l 2 ml 1 ms 12 c n 4 l ...View the full answer

Answered By

Talha Talib
I am a member of IEEE society. As i am a student of electrical engineering badge 17 but beside of this i am also a tutor in unique academy. I teach calculus, communication skills, mechanics and economics. I am also a home tutor. My student Muhammad Salman Alvi is a brilliant A-level student and he performs very well in academics when i start to teach him. His weak point was mathematics but now he is performing well in mathematics. I am a scholarship holder in Fsc as i scored 1017 marks in metric out of 1100. Later on i got scholarship in Punjab Group of Colleges. I got 2nd position in robotics competition in 2018 as my project home automation select for the exhibition in Expocentre.
4.60+
23+ Reviews
62+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Allowed values for the quantum numbers of electrons are as follows: The relationships between n and the shell designations are noted in Table 2.1. Relative to the subshells, l = 0 corresponds to an s...
-
Allowed values for the quantum numbers of electrons are as follows: n = 1, 2, 3, . . . l = 0, 1, 2, 3, . . . , n -1 ml = 0, 1, 2, 3, . . . , l ms = 12 The relationships between n and the shell...
-
The personnel director for a local manufacturing firm has received complaints from the employees in a certain shop regarding what they perceive to be inequities in the annual salary for employees who...
-
1. Arrays - Create a program that asks for 10 integers. Display a navigation menu and perform the following: A. Display the numbers. B. Display the values of even indexes (0th, 2nd, 4th, 6th, etc.)...
-
Discuss the ethical conduct of AIG executives, and how a stronger ethics program might help the company to strengthen the ethics of its corporate culture. When American International Group (AIG)...
-
Suppose that the loan in Problem 5 permits an additional prepayment of principal on any scheduled payment date. Prepare another amortization schedule that reflects a prepayment of $5000 with the...
-
Define supply chain. Explain how disintermediation affects supply chain structure. Explain why the term chain is misleading. Give an example of a supply chain different from the one in Figure CE10-2.
-
Accounting for bonds held to maturity. Murray Company acquired $ 100.000 face value of the outstanding bonds of Campbell Company on January 1. 2008. The bonds pay interest semiannually on June 30 and...
-
Perdon Corporation Manufactures Safes Broe Mobile Cl eam WYPLUS Return to Blackboard Kimmel, Accounting: Tools for Business Decision Making, Se Help System Announcements (1 Unread) I CALCULATOR...
-
Provide the following for the following challenge exercise: a) Income Statement, Gross Margin Standard, year-to-date b) All Journal Entries c) Customer Aged Detail, all customers, with terms at Mar...
-
What type of orbital (i.e., 3s, 4p, . . . ) is designated by these quantum numbers? (a) n = 5, l = 1, me = 0 (b) n = 4, l = 2, me = -2 (c) n = 2, l = 0, me = 0
-
The greatest probability of finding the electron in a small-volume element of the 1s orbital of the hydrogen atom is at the nucleus. Yet the most probable distance of the electron from the nucleus is...
-
Define and describe the process of worrying within the finance domain.
-
Imagine that you are working at Seneca Bank as a Financial Planner. You are working hard to establish your Financial Planning practice and you realize there are many aspects involved in successfully...
-
Consider all of the analytical tools that Marr has presented. Choose 3 and research ways that specific businesses (after 2022 and other than those that Marr has mentioned) have used each of the...
-
First, tell us about an organization that you thoroughly enjoyed working for and explain what the dynamics of that culture was like. Please go into detail. Second, tell us about an organization that...
-
Write down at leastfive items (durable goods, not food) that you purchase and their sourcing (where each is from). For example, a shirt may be assembled in China, designed in the US, and made from...
-
I agree that overtime can be tricky in different countries. As we've seen with piecework, it is hard to implement it in countries where people are not motivated to work past their regular hours, even...
-
Use the graphs of x = ( (t) and y = g(t) to create a graph of y as a function of x.
-
What are the typical record-at-a-time operations for accessing a file? Which of these depend on the current file record?
-
In your opinion, has Carroll done a good job so far of balancing her responsibility with her authority? What does she now have to do in order to sustain or improve that balance and to ensure that her...
-
How difficult was it to come up with a different way of structuring the organization?
-
What would it take to convince the current head of that organization to go along with your suggested changes?
-
1. What is a perquisite? Give some examples of perquisite. What is the residency requirement for a company? State why is input tax credit mechanism the core of Value Added Tax? How does tax evasion...
-
Please answer requirement 2 as well please On January 1, Daughtry Industries issues 4 percent, 10-year bonds payable with a maturity value of $550,000. The bonds sell at 94 and pay interest on...
-
Question 36 Not yet answered Marked out of 2.00 Flag question A newly hired accountant made the following error. IDENTIFY the type of error made and provide the necessary correcting entry for the...

Study smarter with the SolutionInn App


