Write the structures of the isomers you would expect to obtain in the mononitration of m-methoxybenzaldehyde: CHO
Question:
Write the structures of the isomers you would expect to obtain in the mononitration of m-methoxybenzaldehyde:
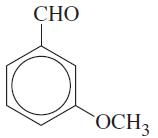
Transcribed Image Text:
CHO OCH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
CHO OCH3 mMethoxybenzaldehyde 3Methyoxybenzeneald...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
You have synthesized a pure molecule with molecular formula SOF4. However, you remember that it has two possible isomers ) and are trying to determine which isomer you have synthesized. Both isomers...
-
Draw structures for (he products you would expect to obtain from reaction of -D-talopyranose with each of the following reagents: (a) NaBH 4 in H 2 O (b) Warm dilute HNO 3 (c) Br 2 , H 2 O (d) CH 3...
-
Ninety kilograms of sodium nitrate is dissolved in 110 kg of water. When the dissolution is complete (at time r = 0), pure water is fed to the tank at a constant rate m (kg/min), and solution is...
-
Though the McDonalds (MCD) menu of hamburgers, cheeseburgers, the Big Mac, Quarter Pounder, Filet-O-Fish, and Chicken McNuggets is easily recognized, McDonalds financial statements may not be as...
-
Give two examples of facility support activities.
-
When Delta Airlines sells tickets for future flights, it debits Cash and credits an account entitled Air Traffic Liability (as opposed to crediting Passenger Revenue Earned). This account, reported...
-
What are the major legal types of carriage? What are the three areas of economic regulation? To which legal type of carriage do they apply? LO.1
-
What items should be included in the audit status report?
-
the management team of Wickersham Brothers incorporated is preparimg its annual financial statements. the statements are complete except for the statement of cash flow. the completed comparative...
-
In the chlorination of CH 4 , some CH 3 CH 2 Cl is obtained as a product. Explain why this should be so.
-
Match the following compounds with the chemical properties given below. Write the structure of the products of the reactions described in (a) to (e). (a) Neutralizes HCl(aq); (b) Neutralizes...
-
16. LO.3 Distinguish between an estate tax and an inheritance tax. a. Do some states impose both? Neither? b. Which, if either, does the Federal government impose?
-
int rFibNum(int a, int b, int n) { if(n == 1) return a; else if( n == 2) return b; else return rFibNum(a,b, n-1) + rFibNum(a, b, n-2); } In the code above; a) how many base cases are there? b) what...
-
Watch the Super Nanny (i.e., Jo Frost) episode "The Orm Family" (Season 1, Episode 3) and answer the following questions. Unless otherwise specified, your answers should focus on Declan (the 3 year...
-
2. Suppose Ford officials were asked to justify their decision. What moral principles do you think they would invoke? Assess Ford's handling of the Pinto from the perspective of each of the moral...
-
2. You have been asked to design the proto-type of an Automatic Grocery Vending Machine 10 (AGVM) for the super store. Automatic Grocery Vending Machine (AGVM) is a machine where different types of...
-
1. What does Porter's 5 Forces analysis strategy do? 2. Do most people agree Why? or disagree with this aspect Why? Here is the reference video, https://www.youtube.com/watch?v=Dfp23xSqpdk 3. What...
-
Connie tosses a fair coin three times. If X = X1 - X2, where X1 counts the number of heads that result and X2 counts the number of tails that result, determine (a) The probability distributions for...
-
Activator rod AB exerts on crank BCD a force P directed along line AB. Knowing that P must have a 100-N component perpendicular to arm BC of the crank, determine (a) The magnitude of the force P, (b)...
-
Schweser Satellites Inc. produces satellite earth stations that sell for $100,000 each. The firms fixed costs, F, are $2 million, 50 earth stations are produced and sold each year, profits total...
-
Now calculate the corporate value. Assume you have just been hired as a business manager of PizzaPalace, a regional pizza restaurant chain. The companys EBIT was $50 million last year and is not...
-
Pettit Printing Company has a total market value of $100 million, consisting of 1 million shares selling for $50 per share and $50 million of 10% perpetual bonds now selling at par. The companys EBIT...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


