Question: a. Complete the following table for an isotopically labeled atom or ion: b. Complete the following table for an isotopically labeled atom or ion: Metal,
a. Complete the following table for an isotopically labeled atom or ion:
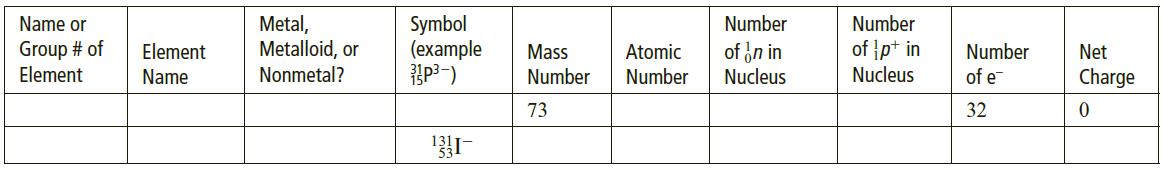
b. Complete the following table for an isotopically labeled atom or ion:
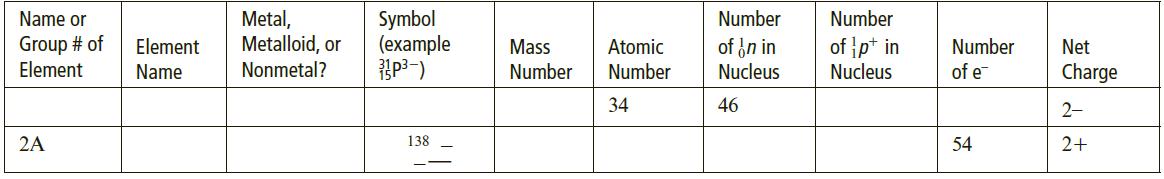
Metal, Metalloid, or Nonmetal? Name or Symbol (example Number Number Group # of Element of in in Nucleus of p+ in Nucleus Element Mass Atomic Number Net Name Number Number of e Charge 73 32
Step by Step Solution
3.51 Rating (161 Votes )
There are 3 Steps involved in it
a For the first species since the net charge is zero the number of protons in the nucleus and the atomic number of the nucleus equals the number of el... View full answer

Get step-by-step solutions from verified subject matter experts


