With the aid of a periodic table (not Figure 9.15), arrange the following in order of increasing
Question:
With the aid of a periodic table (not Figure 9.15), arrange the following in order of increasing electronegativity:
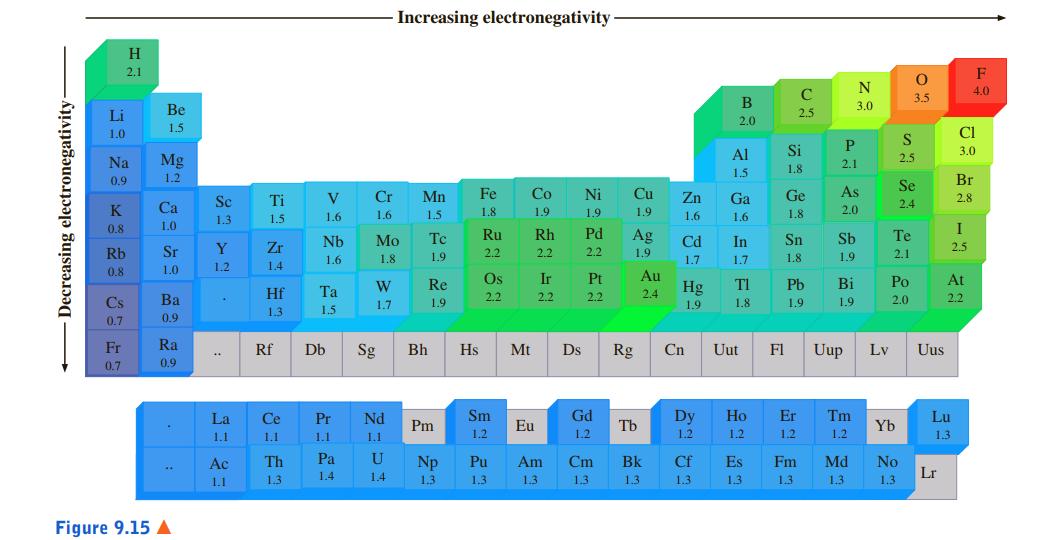
a. Li, Na, Cs
b. B, Be, Li
c. S, Se, Cl
Transcribed Image Text:
Increasing electronegativity H 2.1 F N 4.0 C 3.5 B 3.0 Li Be 2.5 2.0 1.5 1.0 CI Al Si 3.0 Na Mg 2.1 2.5 1.5 1.8 0.9 1.2 Br Se V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Ti 2.4 2.8 K Ca 1.5 2.0 1.6 1.6 1.5 1.8 1.9 1.9 1.9 1.6 1.6 1.8 1.3 1.5 0.8 1.0 I Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Sr Y Zr 2.5 Rb 1.8 1.9 2.2 2.2 2.2 1.9 1.8 1.9 2.1 1.6 1.7 1.7 0.8 1.0 1.2 1.4 Re Os Ir Pt Au TI Pb Bi Po At Hf Ta Hg Cs Ba 2.2 2.2 2.2 2.4 1.9 2.0 2.2 1.7 1.9 1.9 1.8 1.9 1.3 1.5 0.7 0.9 Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Uut F1 Uup Lv Uus 0.7 0.9 La Ce Pr Nd Sm Gd Dy Ho Er Tm Lu Pm Eu Tb Yb 1.1 1.1 1.1 1.1 1.2 1.2 1.2 1.2 1.2 1.2 1.3 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No 1.4 1.4 Lr 1.1 1.3 1.3 1,3 1.3 1.3 1.3 1.3 1.3 1.3 1.3 1.3 Figure 9.15 A Decreasing electronegativity Wn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
a Cs Na Li Electronegativity increases from bottom to ...View the full answer

Answered By

Sumit kumar
Education details:
QUATERNARY Pursuing M.Tech.(2017-2019) in Electronics and Communication Engg. (VLSI DESIGN) from
GNIOT Greater Noida
TERTIARY B.Tech. (2012-2016) in Electronics and Communication Engg. from GLBITM Greater Noida
SECONDARY Senior Secondary School Examination (Class XII) in 2012 from R.S.S.Inter College, Noida
ELEMENTARY Secondary School Examination (Class X) in 2010 from New R.J.C. Public School ,Noida
CERTIFICATION
Summer Training in ‘WIRELESS EMBEDDED SYSTEM’ from ‘XIONEE’ for the six weeks.
EMBEDDED SYSTEM Certificate issued by CETPA INFOTECH for one day workshop.
Certificate of Faculty development program on OPTICAL COMMUNICATION and NETWORKS for one week.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Arrange the following in order of increasing basicity: (a) (b) (c)
-
Arrange the following in order of increasing first ionization energy: Na, Cl, Al, S, and Cs.
-
Arrange the following in order of increasing first ionization energy: F, K, P, Ca, and Ne.
-
In Exercises 1 through 28, differentiate the given function. y = 2x
-
On July 1, a portfolio manager holds $1 million face value of Treasury bonds, the 11 l/4s maturing in about 29 years. The price is 107 14/32. The bond will need to be sold on August 30. The manager...
-
Preventative maintenance tests. The optimal scheduling of preventative maintenance tests of some (but not all) of n independently operating components was developed in Reliability Engineering and...
-
What is the role of Government Business linkages in creating emerging market challengers? Do you think that emerging and transition economies can move to a more hands-off approach with time? LO.1
-
The Sales and Advertising Data (2009) Kramer Smith Case Kramer Smith owns a dry-cleaning service and is thinking about changing his advertising expenditures for the year. He hires you as a consultant...
-
As an auditor for the CPA firm of Hinkson and Calvert, you encounter the following situations in auditing different clients. 1. Novak Corp. is a closely held corporation whose stock is not publicly...
-
Select the net ionic equation for the reaction between sodium chloride and mercury(I) nitrate. 2NaCl( aq ) + Hg 2 (NO 3 ) 2 ( aq ) 2NaNO 3 ( aq ) + Hg 2 Cl 2 ( s ) A. Na + ( aq ) + NO 3 ( aq ) ...
-
Decide which of the following bonds is least polar on the basis of electronegativities of atoms: ClH, SSi, BrAs.
-
Assuming that the atoms form the normal number of covalent bonds, give the molecular formula of the simplest compound of germanium and fluorine atoms.
-
_isthe estimated selling price in the ordinary course of business less the estimated selling costs of completion and the estimated costs necessary to make the sale.
-
1. A corn farmer has observed the following distribution for the number of ears of corn per cornstalk. Ears of Corn Probability 1 2 3 4 .3 .4 .2 .1 Part A: How many ears of corn does he expect on...
-
1. A mass m on a vertical spring with force constant k has an amplitude of A. Using the top of the motion as the origin for both gravitational potential energy and spring potential energy: (a) Find...
-
2. Consider the PDE Utt - Uxx + Ut - Ux = 0 (1) for < < and 0
-
On April 1, 2024, Chardonnay pays an insurance company $12,480 for a two- year fire insurance policy. The entire $12,480 is debited to Prepaid Insurance at the time of the purchase. Record the...
-
Which retailer(s) should represent and sell your product?Why?In terms of their range of distribution coverage, is your retailer intensive, selective and exclusive? Why is this aspect important to...
-
By July 1, 20X2, the market yield on the Akers Company bonds described in had risen to 10%. Required: What was the bonds market price on July 1, 20X2?
-
You are standing on the top of a building and throw a ball vertically upward. After 2 seconds, the ball passes you on the way down, and 2 seconds after that, it hits the ground below. a. What is the...
-
A solution is made up by dissolving 15.0 g Na2CO310H2O in 100.0 g of water. What is the molality of Na2CO3 in this solution?
-
An aqueous solution is 15.0% by mass of copper(II) sulfate pentahydrate, CuSO45H2O. What is the molarity of CuSO4 in this solution at 20C? The density of this solution at 20C is 1.167 g/mL.
-
An aqueous solution is 20.0% by mass of sodium thiosulfate pentahydrate, Na2S2O35H2O. What is the molarity of Na2S2O3 in this solution at 20C? The density of this solution at 20C is 1.174 g/mL.
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


