Given the slightly negative slope of the phase boundary between liquid water and ice in Figure 1.2,
Question:
Given the slightly negative slope of the phase boundary between liquid water and ice in Figure 1.2, how much would the pressure-melting point of ice be depressed at the base of an ice sheet 1 km thick?
Figure 1.2
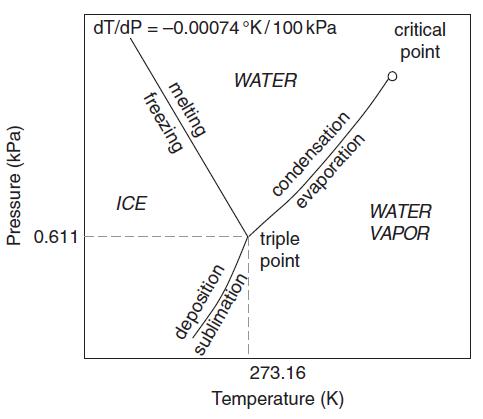
Transcribed Image Text:
Pressure (kPa) 0.611 dT/dP = -0.00074 °K/100 kPa freezing melting ICE WATER deposition sublimation. triple point condensation evaporation 273.16 Temperature (K) critical point WATER VAPOR
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
DTdP 000074...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Geomorphology The Mechanics And Chemistry Of Landscapes
ISBN: 9780521519786
1st Edition
Authors: Robert S. Anderson, Suzanne P. Anderson
Question Posted:
Students also viewed these Sciences questions
-
In Section 11.5 we defined the vapor pressure of a liquid in terms of an equilibrium. (a) Write the equation representing the equilibrium between liquid water and water vapor and the corresponding...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Calculate the difference in molar entropy (a) between liquid water and ice at -5C, (b) between liquid water and its vapour at 95C and 1.00 atm. The differences in heat capacities on melting and on...
-
For this project, you must select an employer organization and research the organizations employee benefits package (plan). After you research the organizations employee benefits package, collect...
-
Consider a bead of mass m that is free to move on a thin, circular wire of radius r. The bead is given an initial speed v 0 , and there is a coefficient of kinetic friction k. The experiment is...
-
Starting from testosterone (Problem 17.48), how would you prepare the followingsubstances? CH (a) (b) C CH - (c) (d) CH CH -
-
8. What accounting and reporting methods are used by investor-venturers in accounting for their joint venture investments? E x E R c I S E S
-
A researcher developed the following multiple regression model to explain the variation in hours worked by married women. H = β0 + β1X1 + β2X2 + β3X3 +...
-
Golden Corporation's current year income statement, comparative balance sheets, and additional information follow. For the year, ( 1 ) all sales are credit sales, ( 2 ) all credits to Accounts...
-
You want to park your bicycle in a bicycle parking area where bike racks are aligned in a row. There are already N bikes parked there (each bike is attached to exactly one rack, but a rack can have...
-
Based upon the 18 O curve, how much colder, in C, is the Earth surface now than at the Eocene Climatic Optimum shown in Figure 1.3? And in F? Figure 1.3 K/T Age (Ma) 5 4 0 10 20 30 40 50 60 70 T...
-
Find parametric equations for the line through P = (2, 5) perpendicular to the line y = 4x 3.
-
Talk with a loan officer at a local bank or credit union. Discuss the approval qualifications for unsecured, secured, and home equity loans and the range of interest rates available with each. Also,...
-
Archer Contracting repaved 50 miles of two-lane county roadway with a crew of six employees. This crew worked 8 days and used \($7,000\) worth of paving material. Nearby, Bronson Construction repaved...
-
An insurance company has the following profitability analysis of its services: The fixed costs are distributed equally among the services and are not avoidable if one of the services is dropped. What...
-
The Scantron Company makes bar-code scanners for major supermarkets. The sales staff estimates that the company will sell 500 units next year for 10,000 each. The production manager estimates that...
-
Determine the following: a. The stockholders equity of a company that has assets of \(\$ 625,000\) and liabilities of \(\$ 310,000\). b. The retained earnings of a company that has assets of \(\$...
-
You are the manager of internal audit for Do-It-All, Ltd., a large, diversified, decentralized manufacturing company. Over the past two years, the information systems function in Do-It-All has...
-
Discussion 1: What issues do you see with this process of buying the computers from John's small computer company? If you were Reid Lewis, how would you respond to Diana when she comes to your office...
-
The tractor is used to lift the 150-kg load B with the 24-mlong rope, boom, and pulley system. If the tractor travels to the right at a constant speed of 4 m/s, determine the tension in the rope when...
-
Propose a plausible synthesis for the following transformation. Enantiomer
-
Using bromobenzene and ethylene oxide as your only sources of carbon, show how you could prepare trans- 1, 2-diphenyloxirane (a racemic mixture of enantiomers). + Enantiomer
-
The S N 2 reaction between a Grignard reagent and an epoxide works reasonably well when the epoxide is ethylene oxide. However, when the epoxide is substituted with groups that provide steric...
-
4) Read the following case carefully and answer the given questions. You have been the finance director of a clothing retailer for ten years. The companys year end is 31st December 2019, and you are...
-
all of the other problems here on chegg don't describe right on how they god the answer. can you make it step by step math to show how you got what and from where and each number to get the answer...
-
D Required information The following Information applies to the questions displayed below.) Diego Company manufactures one product that is sold for $76 per unit in two geographic regions-the East and...

Study smarter with the SolutionInn App


