Consider the binary batch distillation of Example 6.3. For this system, (n)-heptane with (n)-octane at (1 mathrm{~atm}),
Question:
Consider the binary batch distillation of Example 6.3. For this system, \(n\)-heptane with \(n\)-octane at \(1 \mathrm{~atm}\), the average relative volatility is \(\alpha=2.16\) (Treybal, 1980). Using equation (6-105) derived in Problem 6.7, compute the composition of the residue after \(60 \mathrm{~mol} \%\) of the feed is differentially distilled.
Data From Example 6.3:-
Suppose the liquid of Example 6.1 [50 mol% n-heptane (A), 50 mol% n-octane (B)] were subjected to a batch distillation at atmospheric pressure, with 60 mol% of the iquid distilled. Compute the composition of the composited distillate and the residue.
Data From Problem 6.7:-
If equation (6-40) describes the equilibrium relation at constant pressure by use of some average relative volatility \(\alpha\) over the concentration range involved, show that equation (6-11) becomes.
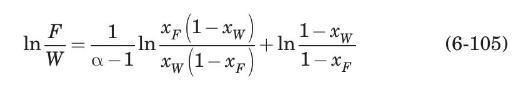

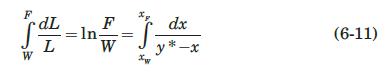
Data From Example 6.1:-
A distillation column with a partial reboiler and a total condenser is being used to separate a mixture of benzene, toluene, and 1,2,3-trimethylbenzene. The feed, 40 mol% benzene, 30 mol% toluene, and 30 mol% 1,2,3-trimethylbenzene, enters the column as a saturated vapor. We desire 95% recovery of the toluene in the distillate and 95% of the 1,2,3-trimethylbenzene in the bottoms. The reflux is returned as a saturated liquid, and constant molar overflow can be assumed. The column operates at a pressure of 1 atm. Find the number of equilibrium stages required at total reflux, and the recovery fraction of benzene in the distillate. Solutions of benzene, toluene, and 1,2,3-trimethylbenzene are ideal.
Step by Step Answer:






