Consider the ternary mixture of Example 6.2 and Problem 6.3. Estimate the temperature, and composition of the
Question:
Consider the ternary mixture of Example 6.2 and Problem 6.3. Estimate the temperature, and composition of the liquid and vapor phases when \(60 \%\) of the mixture has been vaporized at a constant pressure of \(1 \mathrm{~atm}\).
Data From Example 6.2:-
A liquid containing 50 mol% benzene (A), 25 mol% toluene (B), and 25 mol% o-xylene (C) is flash-vaporized at 1 atm and 373 K. Compute the amounts of liquid and vapor products and their composition. These components form ideal mixtures. The vapor pressures of the three components at 373 K are PA = 178.8 kPa, PB = 73.6 kPa, and PC = 26.3 kPa. Therefore, for a total pressure of 1 atm and a temperature of 373 K, mA = 1.765, mB = 0.727, mC = 0.259.
Data From Problem 6.3:-
Modify the Mathcad program of Figure 6.3 for ternary mixtures. Test your program with the data presented in Example 6.2 for a mixture of benzene (A), toluene (B), and \(o\)-xylene (C). Critical temperatures and pressures, and the parameters of the Wagner equation for estimating vapor pressure (equation 6-5) are included in the following table (Reid et al., 1987).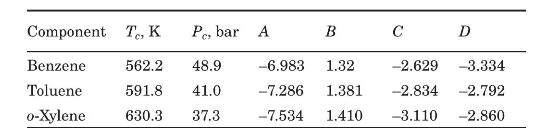
Step by Step Answer:






