(a) Comment why, in Fig. 13.1, the data are presented on a logarithmic scale. What are the...
Question:
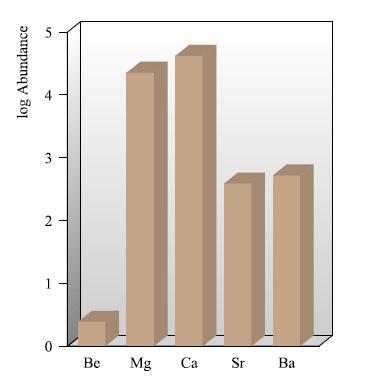
(a) Comment why, in Fig. 13.1, the data are presented on a logarithmic scale. What are the relative abundances of Al (Fig. 13.1) and Mg (Fig. 12.2) in the Earth’s crust?
(b) Show that the changes in oxidation states for elements undergoing redox changes in reaction 13.18 balance.
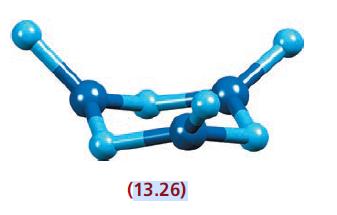
(c) The ion [B3N6]9− in La5(BN3)(B3N6) possesses a chair conformation with each B atom being in an approximately trigonal planar environment (see structure 13.26); B—N bond lengths in the ring are 148 pm, and the exocyclic B—N bond lengths average 143 pm. Draw a set of resonance structures for [B3N6]9−, focusing on those structures that you consider will contribute the most to the overall bonding. Comment on the structures you have drawn in the light of the observed structure of the ion in crystalline La5(BN3)(B3N6).
Figure 13.1.
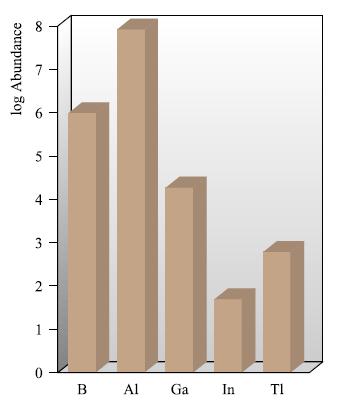
Figure 12.2.
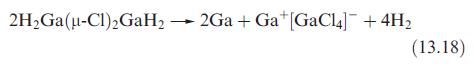
Step by Step Answer:






