Suggest likely products for the following reactions, with the stoichiometries stated: (a) B5 H + Br (b)
Question:
Suggest likely products for the following reactions, with the stoichiometries stated:
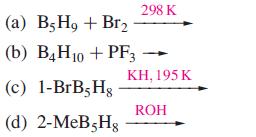
Transcribed Image Text:
(a) B5 H₂ + Br₂ (b) B4 H10 + PF3 (c) 1-BrB5H8 (d) 2-MeB5Hg 298 K KH, 195 K ROH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To predict the likely products of the given reactions we need to consider the reactivity of the reac...View the full answer

Answered By

Akash M Rathod
I have been utilized by educators and students alike to provide individualized assistance with everything from grammar and vocabulary to complex problem-solving in various academic subjects. I can provide explanations, examples, and practice exercises tailored to each student's individual needs, helping them to grasp difficult concepts and improve their skills.
My tutoring sessions are interactive and engaging, utilizing a variety of tools and resources to keep learners motivated and focused. Whether a student needs help with homework, test preparation, or simply wants to improve their skills in a particular subject area, I am equipped to provide the support and guidance they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suggest likely products for the following reactions (which are balanced on the left-hand sides) in liquid NH 3 . How does reaction (d) differ from the behaviour of MeCO 2 H in aqueous solution? (a)...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
(a) Suggest products for the following reactions. (b) PhB(OH) 2 forms dimers in the solid state. Dimers further associate into a 3-dimensional network. Describe how this assembly is likely to arise....
-
Local 54 has retained James Love to represent it in a grievance against Dilated Peoples Optical Inc. The union is grieving the employers decision to exclude from the bargaining unit the position of...
-
Refer to the data given in Problem 1645. The County Board of Representatives believes that if the county conducts a promotional effort costing $20,000 per year, the proposed long runway will result...
-
In Exercises find the curvature and radius of curvature of the plane curve at the given value of x. y = ln x, x = 1
-
Contract costing and job costing are the same.
-
The dollar cost of debt for Coval Consulting, a U.S. research firm, is 7.5%. The firm faces a tax rate of 30% on all income, no matter where it is earned. Managers in the firm need to know its yen...
-
Total Labor Variance Tico Inc. produces plastic bottles. Each bottle has a standard labor requirement of 0.01 hours. During the month of April, 520,000 bottles were produced using 14,000 labor hours...
-
(a) Write balanced equations for the reactions of aqueous Ga + with [I 3 ] , Br 2 , [Fe(CN) 6 ] 3+ and [Fe(bpy) 3 ] 3+ . (b) The 205 Tl NMR spectrum of an acidic solution that contains Tl 3+ and...
-
(a) Comment why, in Fig. 13.1, the data are presented on a logarithmic scale. What are the relative abundances of Al (Fig. 13.1) and Mg (Fig. 12.2) in the Earths crust? (b) Show that the changes in...
-
Suppose you have been presented with selected information taken from the financial statements of Southwest Airlines Co., shown below. Instructions (a) Calculate each of the following ratios for 2014...
-
Required information Use the following information for the Exercises below. (Algo) [The following information applies to the questions displayed below.] Ramirez Company installs a computerized...
-
Reproduced below from Farthington Supply s accounting records is the accounts receivable subledger along with selected general ledger accounts. General Ledger Accounts Receivable Dec. 3 1 / 2 2...
-
James A. and Ella R. Polk, ages 70 and 65, respectively, are retired physicians who live at 3319 Taylorcrest Street, Houston, Texas 77079. Their three adult children (Benjamin Polk, Michael Polk, and...
-
Required information [The following information applies to the questions displayed below.] Shauna Coleman is single. She is employed as an architectural designer for Streamline Design (SD). Shauna...
-
The following are the ratings of men by women in an experiment involving speed dating. Use the given data to construct a boxplot and identify the 5-number summary. 3.0 3.5 4.0 4.5 5.5 5.5 6.5 6.5 6.5...
-
Describe capital budgeting under conditions of risk, risk-adjusted discount rate, and certainty equivalent, and when it would be a good idea to use these methods for considering business risk?
-
A routine activity such as pumping gasoline can be related to many of the concepts studied in this text. Suppose that premium unleaded costs $3.75 per gal. Work Exercises in order. Use the...
-
Determine whether the number of IR and Raman active stretching modes could be used to determine uniquely whether a sample of gas is BF 3 , NF 3 , or ClF 3 .
-
Determine the symmetry elements and assign the point group of (a) NH 2 Cl, (b) CO 3 2 , (c) SiF 4 , (d) HCN, (e) SiFClBrI, (f) BF 4 .
-
Group theory is often used by chemists as an aid in the interpretation of IR spectra. For example, there are four NH bonds in NH 4 + and four stretching modes are possible. There is the possibility...
-
C0 = 10.648148 b) ( 4 Marks ) As of now, Given the above conditions on the option, what is the intrinsic value of the call option? What is the time value of the call option?
-
interest revenue 19,500 retained earning,end 5,000 selling expenses 145,00 prepaid insurance 20,000 loss and disposal of a business (discountied),net 28,000 income from operation 140,000 unearned...
-
cost that do not extend the acid capacity or it's useful life, but merely maintained the assd, or restore it to working order are recorded as losses True or False

Study smarter with the SolutionInn App


