(a) Suggest products for the following reactions. (b) PhB(OH) 2 forms dimers in the solid state. Dimers...
Question:
(a) Suggest products for the following reactions.
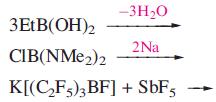
(b) PhB(OH)2 forms dimers in the solid state. Dimers further associate into a 3-dimensional network. Describe how this assembly is likely to arise.
Transcribed Image Text:
-3H₂O 3EtB(OH)2 CIB(NMe 2)2 K[(C₂F5)3BF] + SbF5 2Na
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
a Products of the given reactions 3HO presumably a compound with three water molecules When heated this compound might undergo a dehydration reaction ...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
For the following reactions at constant pressure, predict if H . E, H , E, or H = E. a. 2HF(g) H2(g) + F2(g) b. N2(g) + 3H2(g) 2NH3(g) c. 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g)
-
Nitric acid hydrates have received much attention as possible catalysts for heterogeneous reactions that bring about the Antarctic ozone hole. Worsnop et al. investigated the thermodynamic stability...
-
Aneko Company reports the following ($000s): net sales of $14,800 for 2018 and $13,990 for 2017; end-of-year total assets of $19,100 for 2018 and $17,900 for 2017. Compute its total asset turnover...
-
The management of Iroquois National Bank is considering an investment in automatic teller machines. The machines would cost $124,200 and have a useful life of seven years. The banks controller has...
-
When t = 0, an object is at the point (0, 1) and has a velocity vector v(0) = -i. It moves with an acceleration of a(t) = sin ti - cos tj. Show that the path of the object is a circle.
-
Work-in-progress in contract account consists of (a) work certified and profit carried forward (b) work certified (c) work certified and work uncertified (d) work certified, work uncertified and...
-
Derive the nodal finite-difference equations for the following configurations. (a) Node m, n on a diagonal boundary subjected to convection with a fluid at T and a heat transfer coefficient h. Assume...
-
As the discount rate is increased the NPV of a specific project will
-
The ordering of the relative stabilities of adducts L BH 3 for some common adducts is, according to L: Me 2 O < THF < Me 2 S < Me 3 N < Me 3 P < H. In addition to answering each of the following,...
-
Explain how, during dimerization, each BH 3 molecule acts as both a Lewis base and a Lewis acid.
-
Develop a listing of what you believe are the most important metrics for operations managers. (Hint: Be sure to consider the triple bottom line.) How does each metric support the overall financial...
-
1. Mainland purchased a machine for $85,000 on 1 January 20x7 and assigned it a useful life for 10 years. On 31 March 20x9 it was revalued to $93,000 with no change in useful life. Complete the table...
-
Find the equation of the regression line and identify a characteristic of the data that is ignored by the regression line X 10 8 13 9 11 14 6 4 12 7 5 Y 7.46 6.77 12.74 7.11 7.81 8.84 6.08 5.39 8.15...
-
For each of the following independent cases, fill in the missing amounts in the table: (Indicate the effect of each variance by selecting "F" for favorable, "U" for unfavorable.) Case Direct Labor...
-
All views expressed in this paper are those of the authors and do not necessarily represent the views of the Hellenic Observatory or the LSE George Alogoskoufis Greeces Sovereign Debt Crisis:...
-
Current Attempt in Progress Nash Company is constructing a building. Construction began on February 1 and was completed on December 31. Expenditures were $1,812,000 on March 1, $1,212,000 on June 1,...
-
In what order do direct material costs move through a company's accounts, from purchase to sale? a) Raw Materials > Manufacturing Overhead > Work in Process > Finished Goods > Cost of Goods Sold b)...
-
Write each fraction as a percent. 7 50
-
Use the data in Table 2.7 to calculate the standard enthalpy of the reaction 2 H 2 (g) + O 2 (g) 2 H 2 O(g). The experimental value is 484 kJ. Account for the difference between the estimated and...
-
Use the covalent radii in Table 2.6 to calculate the bond lengths in (a) CCl 4 (177 pm), (b) SiCl 4 (201 pm), (c) GeCl 4 (210 pm). (The values in parentheses are experimental bond lengths and are...
-
What shapes would you expect for the species (a) ClF 3 , (b) lCl 4 , (c) I 3 ?
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


