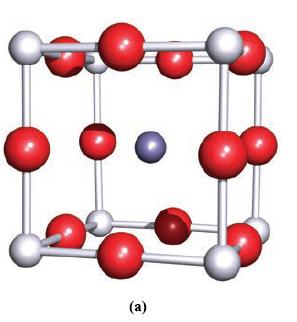
(a) Confirm that the unit cell for perovskite shown in Fig. 6.26a is consistent with the stoichiometry...
Question:
(a) Confirm that the unit cell for perovskite shown in Fig. 6.26a is consistent with the stoichiometry CaTiO3.
(b) A second unit cell can be drawn for perovskite. This has Ti(IV) at the centre of a cubic cell; Ti(IV) is in an octahedral environment with respect to the O2− ions. In what sites must the Ca2+ ions lie in order that the unit cell depicts the correct compound stoichiometry? Draw a diagram to illustrate this unit cell.
Figure 6.26a
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





