(a) Estimate the value of f H(WCl 2 ) assuming it to be an ionic compound....
Question:
(a) Estimate the value of ΔfH°(WCl2) assuming it to be an ionic compound. Comment on any assumptions made. [Data needed in addition to those in Tables 21.1, 22.1 and the Appendices: ΔfH°(CrCl2) = −397 kJ mol−1].
(b) What does your answer to (a) tell you about the likelihood of WCl2 being ionic?
Table 21.1.
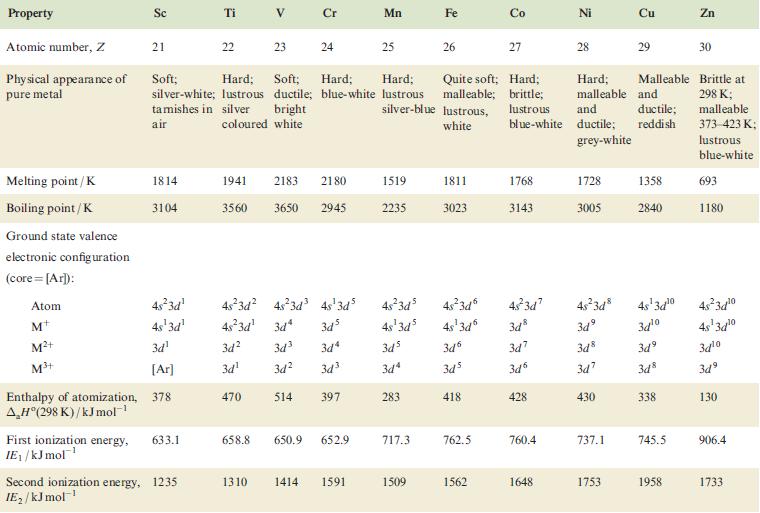
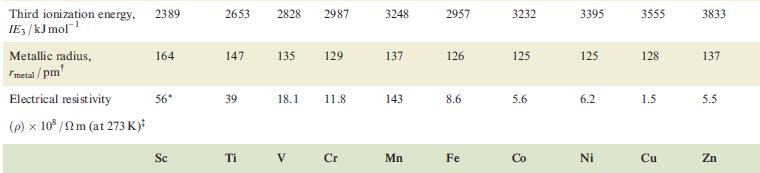
Table 22.1
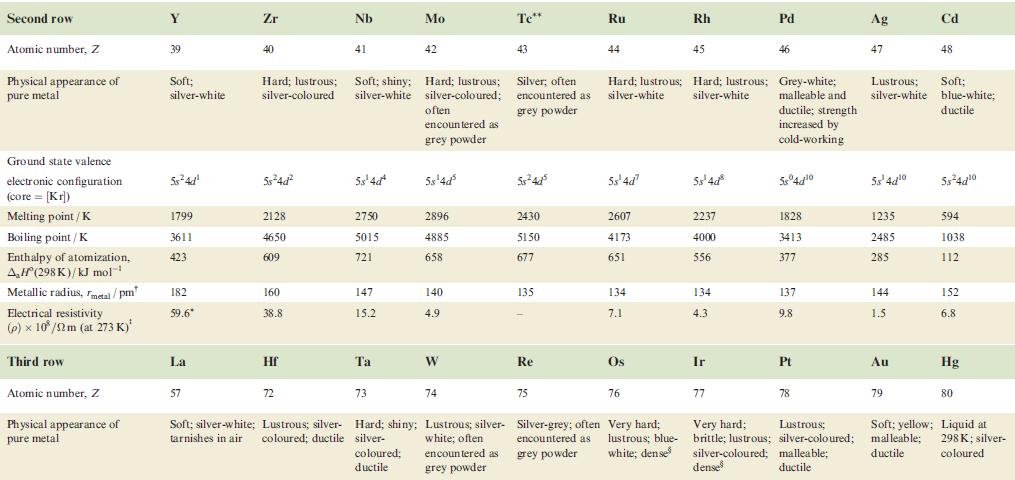
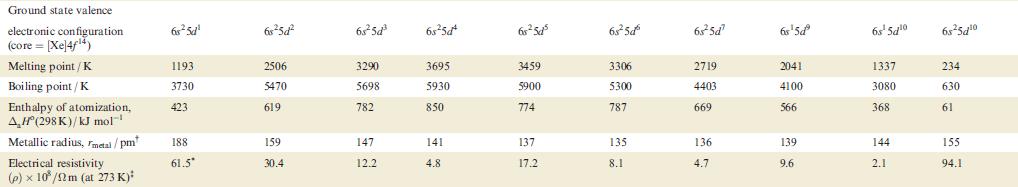
Transcribed Image Text:
Property Atomic number, Z Physical appearance of pure metal Melting point / K Boiling point/K Ground state valence electronic configuration (core=[Ar]): Atom M+ M²+ M³+ Enthalpy of atomization, AH (298 K)/kJ mol-¹ Sc 21 1814 3104 4s²3d¹ 4s¹3d¹ 3d¹ [Ar] 378 First ionization energy, 633.1 IE₁/kJ mol™¹ Ti Second ionization energy, 1235 IE₂/kJ mol-¹ 22 V 23 Cr 24 1941 2183 2180 3560 3650 2945 3d² 3d³ 3d¹ 470 Soft; Hard; Soft; Hard; Hard; Quite soft; Hard; silver-white; lustrous ductile; blue-white lustrous malleable; brittle; tamishes in silver bright silver-blue lustrous, lustrous air coloured white white blue-white 48²3d² 4s²3d³ 4s¹3d5 4s²3d¹3d4 345 3d4 3d² 3d³ 514 397 658.8 650.9 652.9 Mn 1310 1414 1591 25 1519 2235 Fe 717.3 26 1509 1811 3023 3d6 3d5 418 Co 762.5 27 1562 4s²3d³ 48²3d 45-3d7 4s²3d8 4s¹3d³ 4s¹3d6 3d8 зая 3d³ 3d7 348 3d4 3d6 3d7 283 1768 3143 428 760.4 Ni 1648 28 1728 3005 Hard; Malleable and malleable and ductile; ductile; reddish grey-white 430 737.1 Cu 1753 29 1358 2840 4s¹3d¹⁰ 34¹⁰ 3d9 3d8 338 745.5 1958 Zn 30 Brittle at 298 K; malleable 373-423 K; lustrous blue-white 693 1180 4s²3d¹0 4s¹3d¹0 3d¹0 3d⁹ 130 906.4 1733
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To estimate the value of fHWCl2 as if it were an ionic compound ...View the full answer

Answered By

Rehab Rahim
I am well versed in communicating and teaching in areas of all business subjects. I have helped many students in different ways from answering answers to writing their academic papers.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The first quarter of 2008 had not yet ended and Steve Savage already knew the company would surpass the projected $22 million in revenues for the year. He and the management team had doubled sales...
-
Read the comment letter below written by Micron's CFO to the FASB dated June 30, 2004 and answer the following question. Director of Major Projects Financial Accounting Standards Board 401...
-
In our development of consumer theory, we made a big point about the fact that neoclassical economics does not put much stock in the idea of cardinally measuring utility (in terms of units of...
-
Night By Elie Wiesel The Holocaust - Why did the members of Sighets Jewish community refuse to believe their horrible situation? (Moshe the Beadle and Madame Schachter portending the horrors that...
-
Expando, Inc., is considering the possibility of building an additional factory that would produce a new addition to its product line. The company is currently considering two options. The first is a...
-
Find the lengths of the curve. x = 8 cost y = 8 sin t 0 t /2 + 8t sin t 8t cos t,
-
Vino Veritas Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,000 cases ofwine at a price of 200 euros per case. The total purchase price is 200,000...
-
Do some reading in periodicals and/or on the Internet to find out more about the Sarbanes-Oxley Acts provisions for companies. Select one of those provisions, and indicate why you think financial...
-
Total costs for Locke & Company at 160,000 units are $309,000, while total fixed costs are $165,000. The total variable costs at a level of 300,000 units would be (Round intermediate calculations to...
-
Apply the translation theorem to find the Laplace transforms of the functions in Problems 1 through 4. f(t) = e-1/2 cos 2 (1 ) gr
-
Briefly discuss trends in (a) Metallic radii and (b) Values of a H(298 K) for the d-block metals.
-
A gas of O 2 molecules is in the equilibrium state with number density n and absolute temperature T. Calculate the average value of the reciprocal of the particle velocity, that is, < 1/v >.
-
A parent acquires all of the stock of a subsidiary for $40 million in cash. The subsidiarys books report the following account balances at the date of acquisition (in trial balance format)....
-
1. Given: The sign for the Inn of the Prancing Pony in Bree-yes, it comes in pints-is fixed on the end of a beam of length 5L. If the sigh deflects too much then Gandalf will hit his head when he...
-
Q21) Add positive and negative charges as shown in the diagram below. Use the arrows of the simulation to guide you in drawing continuous electric field lines around and in between the three charges....
-
When 10.1 g CaO is dropped into a styrofoam coffee cup containing 157 g H2O at 18.0C, the temperature rises to 35.8C. Calculate the enthalpy change of the following reaction in kJ/mol CaO. Assume...
-
4-12. Sometimes heterogeneous chemical reactions take place at the walls of tubes in which reactive mixtures are flowing. If species A is being consumed at a tube wall because of a chemical reaction,...
-
Refer to PB1-1. Assume that you are the president of Aerospace Explorations. At the end of the first year of operations (December 31, 2014), the following financial data for the company are...
-
One study found that the elderly who do not have children dissave at about the same rate as the elderly who do have children. What might this finding imply about the reason the elderly do not dissave...
-
State the effect on the rate of dissociatively activated reactions of Rh(III) complexes of (a) An increase in the overall charge on the complex, (b) Changing the leaving group from NO 3 to Cl , (c)...
-
Put in order of increasing rate of substitution by H 2 O the complexes (a) [Co(NH 3 ) 6 ] 3+ , (b) [Rh(NH 3 ) 6 ] 3+ , (c) [Ir(NH 3 ) 6 ] 3+ , (d) [Mn(OH 2 ) 6 ] 2+ , (e) [Ni(OH 2 ) 6 ] 2+ .
-
Predict the products of the following reactions: (a) [Pt(PR 3 ) 4 ] 2+ + 2Cl (b) [PtCl 4 ] 2 + 2PR 3 (c) cis-[Pt(NH 3 ) 2 (py) 2 ] 2+ + 2Cl
-
thumbs up if correct A stock paying no dividends is priced at $154. Over the next 3-months you expect the stock torpeither be up 10% or down 10%. The risk-free rate is 1% per annum compounded...
-
Question 17 2 pts Activities between affiliated entities, such as a company and its management, must be disclosed in the financial statements of a corporation as O significant relationships O segment...
-
Marchetti Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,000 cases of wine at a price of 200 euros per case. The total purchase price is 200,000...

Study smarter with the SolutionInn App


