(a) Explain the forms of the d orbital splitting diagrams for trigonal bipyramidal and square pyramidal complexes...
Question:
(a) Explain the forms of the d orbital splitting diagrams for trigonal bipyramidal and square pyramidal complexes of formula ML5 shown in Fig. 20.11.
(b) What would you expect concerning the magnetic properties of such complexes of Ni(II)?
Figure 20.11.
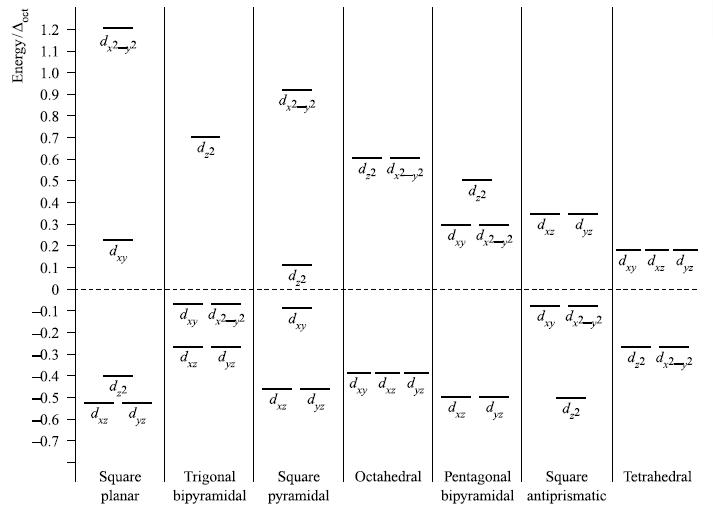
Transcribed Image Text:
Energy/oct 1.2 1.1 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 -0.1 -0.2 -0.3 -0.4 -0.5 -0.6 -0.7 d22 d xy Toe *9/29/ d PR d yz XZ d.2 dxy yz Square Trigonal Square planar bipyramidal pyramidal d_2 d2,2 d d xy XZ yz Octahedral d₂ dy d₂2_2 dre dyz de yz dd22 xy d2 xy d d yz d2 d22 Pentagonal Square Tetrahedral bipyramidal antiprismatic
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
a Trigonal bipyramidal In a molecule having a shape of trigonal bipyramidal Two atoms will be situat...View the full answer

Answered By

Madhur Jain
I have 6 years of rich teaching experience in subjects like Mathematics, Accounting, and Entrance Exams preparation. With my experience, I am able to quickly adapt to the student's level of understanding and make the best use of his time.
I focus on teaching concepts along with the applications and what separates me is the connection I create with my students. I am well qualified for working on complex problems and reaching out to the solutions in minimal time. I was also awarded 'The Best Tutor Award' for 2 consecutive years in my previous job.
Hoping to get to work on some really interesting problems here.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The energy-level diagram in Figure 9.36 shows that the sideways overlap of a pair of p orbitals produces two molecular orbitals, one bonding and one anti-bonding. In ethylene there is a pair of...
-
The following table shows the stream of income produced by several different assets. In each case, P1 P2, and P3 are the payments made by the asset at the end of years 1, 2, and 3. a. For each asset,...
-
Ques 1: What is the major complaint by firms concerning the Sarbanes-Oxley act of 2012? A. the legislative maximum allowable compensation for a CEO. B. the legal requirement to disclose project...
-
The adjusted trial balance of Silver Sign Company follows: Requirements 1. Assume Silver Sign Company has a January 31 year end. Journalize Silvers closing entries at January 31. 2. How much net...
-
An electronics company makes communications devices for military contracts. The company just completed two contracts. The navy contract was for 2,300 devices and took 25 workers two weeks (40 hours...
-
Find the absolute maximum and minimum values of on the region R. (x, y) = x 3 + 3xy + y 3 + 1 R: The square region enclosed by the lines x = 1 and y = 1
-
CI0.5. Do you consider the direct method to be more informative than the indirect method of presenting cashflow from operations?
-
A Styrofoam bucket of negligible mass contains 1.75 kg of water and 0.450 kg of ice. More ice, from a refrigerator at -15.0oC, is added to the mixture in the bucket, and when thermal equilibrium has...
-
Midwest Fabricators Inc. is considering an investment in equipment that will replace direct labor. The equipment has a cost of $97,000 with a $8,000 residual value and a ten-year life. The equipment...
-
Outline how you would apply crystal field theory to explain why the five d-orbitals in an octahedral complex are not degenerate. Include in your answer an explanation of the barycentre.
-
(a) Which of the following octahedral complexes are chiral: cis-[CoCl 2 (en) 2 ] + , [Cr(ox) 3 ] 3 , trans-[PtCl 2 (en) 2 ] 2+ , [Ni(phen) 3 ] 2+ , [RuBr 4 (phen)] , cis-[RuCl(py)(phen) 2 ] + ? (b)...
-
Distinguish between the qualities of relevance and reliability.
-
How could civil engineers contribute to space debris management and cleanup?
-
In what ways can the principles of resilient infrastructure be applied to design urban systems capable of withstanding natural disasters, and how do these principles contribute to the overall safety...
-
What are local variables and global variables in Python?
-
When to use a tuple vs list vs dictionary in Python? Explain some benefits of Python
-
What is Lambda Functions in Python? How do I modify a string in python?
-
Rumsford Inc.'s financial statements for 2019 indicate the following account balances: Net sales ........................................................ $256,340 Cost of goods sold...
-
A fast-food restaurant averages 150 customers per hour. The average processing time per customer is 90 seconds. a. Determine how many cash registers the restaurant should have if it wishes to...
-
(R)-Limonene is found in many citrus fruits, including oranges and lemons: Draw the structures and identify the relationship of the two products obtained when (R)-limonene is treated with excess...
-
Consider the structures of cis-decalin and trans-decalin: (a) Which of these compounds would you expect to be more stable? (b) One of these two compounds is incapable of ring flipping. Identify it...
-
Atorvastatin is sold under the trade name Lipitor and is used for lowering cholesterol. Annual global sales of this compound exceed $13 billion. Assign a configuration to each chirality center in...
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


