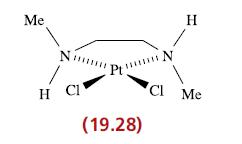
(a) Explain why complex 19.28 is chiral. (b) In each of the following reactions, the left-hand sides...
Question:
(a) Explain why complex 19.28 is chiral.
(b) In each of the following reactions, the left-hand sides are balanced. Suggest possible products and give the structures of each complex formed.
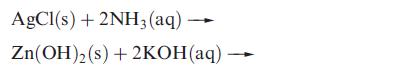
(c) What type of isomerism relates the Cr(III) complexes [Cr(en)3][Cr(ox)3] and [Cr(en)(ox)2][Cr(en)2(ox)]?
Transcribed Image Text:
Me H CI ******** (19.28) H 1 CI Me
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
a In order for a molecule to be chiral it must have a nonsuperimposable mirror image Complex 1928 contains a central metal atom lets assume its a tran...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Sketch a graph of f(t) = e t on an arbitrary interval [a, b]. Use the graph and compare areas of regions to prove that eb ea e + eb ela+b)/2 < b a 2
-
Solve the linear programming problems in Problem by applying the simplex method to the dual problem. Minimize C = 7x1 + 5x2 %3D subject to X1 + x2 2 4 X - 2x, 2 -8 -2x, + x, 2 -8 | X1, X2 2
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
The following Excel output summarizes the results of an analysis of variance experiment in which the treatments were three different hybrid cars and the variable measured was the miles per gallon...
-
What do we mean when we say productivity is a relative measure?
-
What four different areas must be examined when trying to detect financial statement fraud? please provide answers in detailed explanation with references
-
E10.10. Free Cash Flow for Kimberley-Clark Corporation (Medium) Below are summary numbers from reformulated balance sheets for 2007 and 2006 for Kimberly-Clark Corporation, the paper products...
-
On January 3, 2014, Speedway Delivery Service purchased a truck at a cost of $ 65,000. Before placing the truck in service, Speedway spent $ 4,000 painting it, $ 2,500 replacing tires, and $ 8,000...
-
Question Content Area The primary difference between the periodic and perpetual inventory systems is that a a.periodic system keeps a record showing the inventory on hand at all times b.periodic...
-
Show that the trigonal bipyramid, square-based pyramid, square antiprism and dodecahedron belong to the point groups D 3h , C 4v , D 4d and D 2d respectively.
-
Consider the following reaction in which [P 3 O 10 ] 5 (see Fig. 15.21) displaces the carbonate ion to give a mixture of linkage isomers: (a) Suggest possible coordination modes for the [P 3 O 10 ] 5...
-
What is a relation schema? What is the difference between a relation, a relation schema, and a relational schema?
-
Using the figure below, draw the FBD , ?Shear Force and Bending Moment diagrams and find the maximum internal moment for the beam shown. 10 kNm 10 kN 3 m
-
Suppose the goods market is: Y = 1800 - 100i and the LM curve Y = 500 +591, where x is the last digit of your ID number. Determine the equilibrium income (Y), interest rate (i). Explain the role of...
-
Complete the chart: [1] Length, L (m) Period, T (s) LogL LogT 0.10 0.63 0.20 0.90 0.30 1.00 0.40 1.27 0.50 1.42 0.60 1.55 0.70 1.68 0.80 1.80 0.90 1.90 1.00 2.02 Plot the data T vs L. [4 (title, axes...
-
A solid metal sphere with a diameter of 2 cm and a mass of 8 g is used for the following heat transfer experiments. You can assume the temperature throughout the inside of the metal sphere is...
-
2) A car 1200 kg is in a skid Force of friction 3200N and force of air resistance 1600N. Find the following i) The net force and acceleration of the car ii) The time required to stop from 95 km/hr...
-
Refer to Steele's financial statements in Exercise 12-76 and the information below. At January 1, 2018, total stockholders equity was $2,083,122 and there was no preferred stock. Required: 1. Compute...
-
Outline a general process applicable to most control situations. Using this, explain how you would develop a system to control home delivery staff at a local pizza shop.
-
The following reaction does not produce the desired product, but does produce a product that is a constitutional isomer of the desired product. Draw the product that is obtained, and propose a...
-
In the compound below, identify all carbon atoms that are electron deficient (δ+) and all carbon atoms that are electron rich (δ-). Justify your answer with resonance...
-
In each case below, identify the acid and the base. Then draw the curved arrows showing a proton transfer reaction. Draw the products of that proton transfer, and then predict the position of...
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


