(a) Figure 22.37 shows eight corner-sharing ReO 6 octahedra in the solid state structure of ReO 3...
Question:
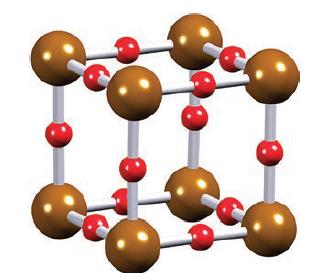
(a) Figure 22.37 shows eight corner-sharing ReO6 octahedra in the solid state structure of ReO3. From this, derive a diagram to show the unit cell of ReO3. Explain the relationship between your diagram and that in Fig. 21.5, and confirm the stoichiometry of the oxide from the unit cell diagram.
(b) A qualitative test for [PO4]3− is to add an excess of an acidified aqueous solution of ammonium molybdate to an aqueous solution of the phosphate. A yellow precipitate forms. Suggest a possible identity for the precipitate and write an equation for its formation.
Figure 23.37
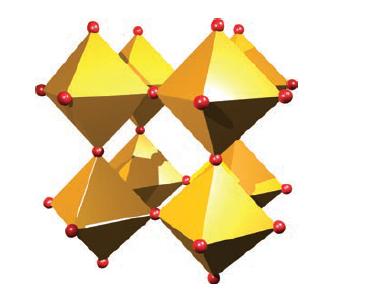
Figure 21.5.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





