(a) In aqueous solution at pH 0, Mn 3+ disproportionates to MnO 2 and Mn 2+ ....
Question:
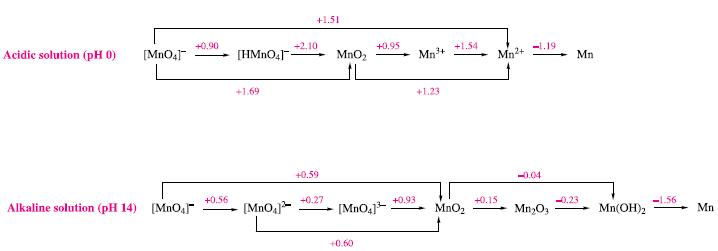
(a) In aqueous solution at pH 0, Mn3+ disproportionates to MnO2 and Mn2+. Write equations for the two half-reactions involved in this process.
(b) Use Fig. 8.2 to obtain values of Eº for the half-equations in part (a).
(c) Determine Eºcell and a value of ΔGº(298 K) for the disproportionation of Mn3+ (aq) at pH 0. Write an equation to which this value of ΔGº(298 K) refers.
Figure 8.2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





