Briefly discuss the trends in boiling points and values of vap H listed in Table 17.2
Question:
Briefly discuss the trends in boiling points and values of ΔvapH° listed in Table 17.2 for the hydrogen halides.
Table 17.2.
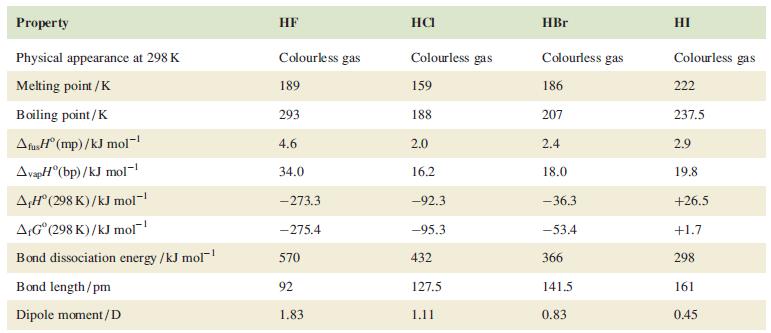
Transcribed Image Text:
Property Physical appearance at 298 K Melting point /K Boiling point/K AfusH (mp)/kJ mol-¹ AvapH (bp)/kJ mol-¹ A,Hº (298 K)/kJ mol-¹ A,Gº (298 K)/kJ mol Bond dissociation energy /kJ mol-¹ Bond length/pm Dipole moment/D HF Colourless gas 189 293 4.6 34.0 -273.3 -275.4 570 92 1.83 HCI Colourless gas 159 188 2.0 16.2 -92.3 -95.3 432 127.5 1.11 HBr Colourless gas 186 207 2.4 18.0 -36.3 -53.4 366 141.5 0.83 HI Colourless gas 222 237.5 2.9 19.8 +26.5 +1.7 298 161 0.45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
From the data provided in Table 172 for the hydrogen halides HF HCl HBr HI we can observe the following trends in boiling points and values of vapH Bo...View the full answer

Answered By

Surendar Kumaradevan
I have worked with both teachers and students to offer specialized help with everything from grammar and vocabulary to challenging problem-solving in a range of academic disciplines. For each student's specific needs, I can offer explanations, examples, and practice tasks that will help them better understand complex ideas and develop their skills.
I employ a range of techniques and resources in my engaged, interesting tutoring sessions to keep students motivated and on task. I have the tools necessary to offer students the support and direction they require in order to achieve, whether they need assistance with their homework, test preparation, or simply want to hone their skills in a particular subject area.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For the hydrogen halides and the noble gases, we have the following boiling points: Halogen Family, C Noble Gases, C HF, 19 ....... Ne, 246 HCl, 115 Ar, 186 HBr, 67 .. Kr, 152 HI, 35 ...... Xe, 108...
-
The Gap Inc. is a global specialty retailer operating stores selling casual apparel, personal care, and other accessories for men, women, and children under The Gap, Banana Republic, and Old Navy...
-
List and briefly discuss the trends impacting the future of retailing.
-
Conduct some additional research to learn more about Fabletics. How is Fabletics meeting customer needs through its value delivery network? What controversy surrounds the company? What type of...
-
Boeing Company is the largest manufacturer of commercial aircraft in the United States and is a major employer in Seattle, Washington. Explain why each of the following individuals or organizations...
-
The following information pertains to JAE Corporation at January 1, Year 1: Common stock, $8 par, 11,000 shares authorized, 2,200 shares issued and outstanding Paid-in capital in excess of par,...
-
Future Value of Advanced Training. Joshua Kelly estimates that taking some classes would result in earning $3,800 more a year for the next 30 years. Based on an annual interest rate of 4 percent,...
-
Total tuition is $3050 for full-time students taking 12 credits or more and $125 per credit hour for part-time students (taking less than 12 credits). Use a function to determine the correct tuition...
-
QUESTION 5 Assume Jay faces the following tax schedule: Taxable Income Marginal Tax rate (in %) Up to $50,000 15 $50,000-$75,000 25 $75,000-$100,000 34 $100,000-$335,000 39 Compute Jay's tax...
-
The [Se 4 ] 2+ ion has D 4h symmetry and the SeSe bond lengths are equal (228 pm). (a) Is the ring in [Se 4 ] 2+ planar or puckered? (b) Look up a value of r cov for Se. What can you deduce about the...
-
For a given atom Y, the YF bond is usually stronger than the corresponding YCl bond. An exception is when Y is oxygen (Table 16.2). Suggest a reason for this observation. Table 16.2. 0-0 146 S-S 266...
-
Which of the following items is not considered to be one of the seven Ms of management? a. Manpower b. Money c. Machines d. Methods e. Materials f. Minutes g. Mission
-
4. Write short notes on Wiener Filtering.
-
1.Explain Histogram processing
-
2. Explain Spatial Filtering ?
-
3. Explain the Geometric Transformations used in image restoration. 4.Describe homomorphic filtering
-
5.Explain the different Noise Distribution in detail. UNIT I V 1. What is segmentation? 2. Write the applications of segmentation. 3. What are the three types of discontinuity in digital image? 4....
-
Discuss what you think are the key elements of Amazon's success and how transferrable these elements are to other organizations
-
Use the graphs of f and g to graph h(x) = (f + g) (x). To print an enlarged copy of the graph, go to MathGraphs.com. 1. 2. y 24 8. 2. -2 -2 4 6
-
(a) Describe the two classes of inorganicorganic nanocomposites based on their bonding types. (b) Give one example of a nanocomposite in each class.
-
Discuss shape selectivity with respect to catalytic processes involving zeolites including mechanisms involving reactant, transition state, and product selectivity.
-
(a) What is the relevance of self-assembly to the fabrication of nanomaterials? (b) What role will it play in nanotechnology?
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


