For a given atom Y, the YF bond is usually stronger than the corresponding YCl bond. An
Question:
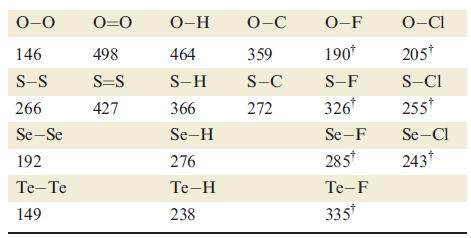
For a given atom Y, the Y—F bond is usually stronger than the corresponding Y—Cl bond. An exception is when Y is oxygen (Table 16.2). Suggest a reason for this observation.
Table 16.2.
Transcribed Image Text:
0-0 146 S-S 266 Se-Se 192 Te-Te 149 0=0 498 S=S 427 O-H 464 S-H 366 Se-H 276 Te-H 238 O-C 359 S-C 272 O-F 190* S-F 326* Se-F 285* Te-F 335* O-CI 205* S-CI 255* Se-Cl 243†
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
The reason for the observation that the YF bond is stronger than the corresponding YCl bond for most ...View the full answer

Answered By

Surendar Kumaradevan
I have worked with both teachers and students to offer specialized help with everything from grammar and vocabulary to challenging problem-solving in a range of academic disciplines. For each student's specific needs, I can offer explanations, examples, and practice tasks that will help them better understand complex ideas and develop their skills.
I employ a range of techniques and resources in my engaged, interesting tutoring sessions to keep students motivated and on task. I have the tools necessary to offer students the support and direction they require in order to achieve, whether they need assistance with their homework, test preparation, or simply want to hone their skills in a particular subject area.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When a beam of atoms emerges from an oven at the absolute temperature T, the most probable de Broglie wavelength for a given atom is In this expression, m is the mass of an atom, and k is Boltzmann's...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
Figure shows a cycle consisting of five paths: AB is isothermal at 300 K, BC is adiabatic with work = 5.0 J, CD is at a constant pressure of 5 atm, D E is isothermal, and EA is adiabatic with a...
-
Identify several ways in which you currently use accounting information in your life as a student. Also identify several situations in which, while you are still a student, you might be required to...
-
Compute the contamination and propagation delays of the circuit in Figure 15.18 from flip-flop D to the output. Assume that the delay through each gate is 10 ps. Data in Figure 15.18 t=15 A t=30 t=5...
-
Comparing the Value of a Career Change. Marla Opper currently earns $50,000 a year and is offered a job in another city for $56,000. The city she would move to has 8 percent higher living expenses...
-
The percentage of individual investors portfolios committed to stock depends on the state of the economy. The following table reports the percentage of stocks in a portfolio for nine quarters: a. Use...
-
discussion question Question: What are SMART Goals? How can they be utilized to assess and improve employee performance
-
Briefly discuss the trends in boiling points and values of vap H listed in Table 17.2 for the hydrogen halides. Table 17.2. Property Physical appearance at 298 K Melting point /K Boiling point/K...
-
[NS 2 ][SbF 6 ] reacts with nitriles, RCN, to give [X] [SbF 6 ] where [X] + is a cycloaddition product. Propose a structure for [X]+ and show that it is a 6-electron system. Do you expect the ring to...
-
Let R be a commutative ring with identity and let ,g e R[[xJJ. Denote by In , the "' initial degree of (that is, the smallest n such that a n 0, where Show that (a) In ( + g) min (In , In g). (b)...
-
6. What are the two properties used for establishing similarity of edge pixels? 7. What is edge? 8. Give the properties of the second derivative around an edge? 9. Define Gradient Operator? 10. What...
-
14. Define pattern. , 15. Define pattern class. 16. List the three pattern arrangements. 17. Give the decision-theoretic methods. 18. Define the training pattern and training set. 19. Define training...
-
1. Write short notes on image segmentation. 2. Write short notes on edge detection 3.Write Short notes on edge linking by local processing.
-
4. Write short notes on the applications of artificial neural networks in image processing.
-
What are the functions of a finance manager of a small firm?
-
What roles do the World Health Organization (WHO) and Center for Disease Control (CDC) have in monitoring the national and world health?
-
In Exercises 1-2, rewrite each verbal statement as an equation. Then decide whether the statement is true or false. Justify your answer. 1. The logarithm of the difference of two numbers is equal to...
-
Aluminosilicate surfaces in zeolites act as strong Brnsted acids, whereas silica gel is a very weak acid. (a) Give an explanation for the enhancement of acidity by the presence of Al 3+ in a silica...
-
(a) Give two examples of applications of quantum wells. (b) Describe why quantum wells are used and if either molecular materials or traditional solid-state materials can exhibit similar properties....
-
Propose formulas for structures that would be isomorphous with SiO 2 and zeolites of the same stoichiometry involving Al, P, B, and Zn, or mixtures thereof, replacing Si.
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.

Study smarter with the SolutionInn App


