Comment on the modes of bonding of the ligands in the Mn(II) complexes listed at the end
Question:
Comment on the modes of bonding of the ligands in the Mn(II) complexes listed at the end of Section 21.8, drawing attention to any conformational restrictions.
Data from Section 21.8
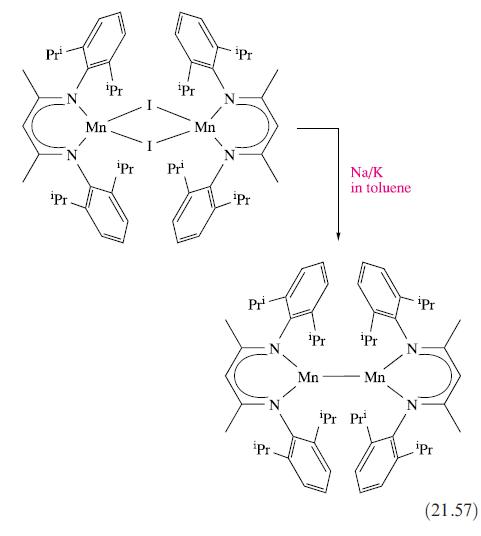
Transcribed Image Text:
Pr Pr iPr Mn iPr iPr Pr¹ Mn Pr ¹Pr Pr N iPr ¹Pr Mn Na/K in toluene Pr Mn iPr Pr Pr ipr (21.57)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
In order to comment on the modes of bonding of the ligands in the MnII complexes listed in equation 2157 we first need to understand the abbreviations ...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
(a) Confirm that H 2 Os 3 (CO) 11 has sufficient valence electrons to adopt a triangular metal framework. Do the modes of bonding of the CO and H ligands affect the total valence electron count?...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
List and describe the payment options (terms of sale) that can be applied to domestic U.S. shipments.
-
The following represents a project that should be scheduled using CPM: a. Draw the network. b. What is the critical path? c. What is the expected project completion time? d. What is the probability...
-
A process to produce Product "A" has four sequential steps. Step one takes 12 minutes, step two takes 8 minutes, step three takes 6 minutes and step four takes 10 minutes. The business operates 8...
-
IAS 1, Presentation of Financial Statements, does not provide guidance with respect to which of the following? LO4 a. The statements that must be included in a complete set of financial statements....
-
Leslies Burgers operates and franchises fast-food restaurants specializing in grilled hamburgers and chicken sandwiches. The 2012 and 2013 income statements are as follows (in $000s): Required a....
-
Problem 18-2A Jorge Company bottles and distributes B-uite, a diet soft drink. The beverage is sold for 50 cents per 16-ounce bottle to retailers, who charge customers 75 cents per bottle for the...
-
(a) Which of the following complexes would you expect to suffer from a JahnTeller distortion: [CrI 6 ] 4 , [Cr(CN) 6 ] 4 , [CoF 6 ] 3 and [Mn(ox) 3 ] 3 ? Give reasons for your answers. (b) [Et 4 N] 2...
-
Values of oct for [Ni(OH 2 ) 6 ] 2+ and high-spin [Mn(OH 2 ) 6 ] 3+ have been evaluated spectroscopically as 8500 and 21000 cm 1 respectively. Assuming that these values also hold for the...
-
C.F. Jordon Construction Services has operated for the last 26 years in a northern U.S. state where the state income tax on corporate revenue is 6% per year. C.F. Jordon pays an average federal tax...
-
Turn this information into an excel sheets with the excel formulas being shown P12.2 (LO 1, 2) (Liability Entries and Adjustments) Listed below are selected transactions of Schultz Department Store...
-
1. Consider an undirected random graph on the set of four vertices {A, B, C, D} such that each of the 4 2 = 6 potential edges exists with probability 0.2, independently of the presence/absence of any...
-
Basic Net Present Value Analysis Jonathan Butler, process engineer, knows that the acceptance of a new process design will depend on its economic feasibility. The new process is designed to improve...
-
Determine the support reactions at the smooth collar A and the normal reaction at the roller support B. 800 N 600 N B 0.8 m 0.4 m 0.4 m 0.8 m
-
A plant hopes to cool a steam line by sending it through a throttling valve to expand it to atmospheric pressure. The steam enters the valve at 550C and 250 bar. The expansion in the valve happens so...
-
Nadal Corporation purchased 10,000 shares of Cutler Inc.'s common stock, on January 1, 2019, for $100,000. During 2019, Cutler declared and paid cash dividends to Nadal in the amount of $8,000....
-
Phosgene, COCl2, is a toxic gas used in the manufacture of urethane plastics. The gas dissociates at high temperature. At 400oC, the equilibrium constant Kc is 8.05 104. Find the percentage of...
-
Identify the structure of the starting alkene in each of the following cases: a. b. c. 1) O3 C3H14 2) DMS C10H16 1) 03 2) DMS
-
When 2-methylpropane is treated with bromine in the presence of UV light, one product predominates. (a) Identify the structure of the product. (b) Draw the structure of the expected minor product....
-
Identify the most electronegative element in each of the following compounds: a) CH 3 OCH 2 CH 2 NH 2 b) CH 2 ClCH 2 F c) CH 3 Li
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


