Determine the g values of the EPR spectrum shown in Fig. 8.57, measured for a frozen sample
Question:
Determine the g values of the EPR spectrum shown in Fig. 8.57, measured for a frozen sample using a microwave frequency of 9.43 GHz.
Figure 8.57
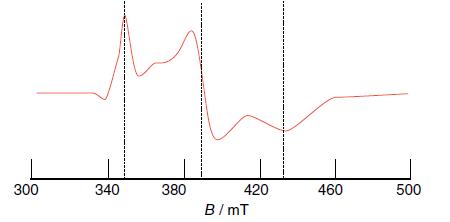
Transcribed Image Text:
300 340 380 420 B/mT 460 500
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The gvalues of the EPR spectrum shown in Fig 857 can be determined using the following equation hv g ...View the full answer

Answered By

KIRAN P
I have M TECH in Chemical Engineering from one of India's best Institute NIT TRICHY and have more than 10 years experience in Chemical industry.Used to teach students during leisure times
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
A researcher wanted to find out if there was difference between older movie goers and younger movie goers with respect to their estimates of a successful actors income. The researcher first...
-
The following table gives the systolic blood pressure (SBP), body size (QUET), age (AGE), and smoking history (SMK = 0 if a nonsmoker, SMK = 1 if a current or previous smoker) for a hypothetical...
-
Helium gas is throttled steadily from 500 kPa and 70C. Heat is lost from the helium in the amount of 2.5 kJ/kg to the surroundings at 25C and 100 kPa. If the entropy of the helium increases by 0.25...
-
Presented below is financial information related to the 2015 operations of Louisa Cruise Company. Maintenance and repairs expense .......$ 92,000 Utilities expense ............... 10,000 Salaries and...
-
In a book entitled The Art of Choosing, author Sheena Iyengar shares a six-step process for helping you decide between how you spend your time at work and/or school, summarized here. 1. Record all...
-
Calculate the degree of financial gearing for a business and explain its significance.
-
On June 1, Maui Travel Agency, Inc., was established. The following transactions were completed during the month. 1. Stockholders invested $40,000 cash, receiving common stock in exchange. 2. Paid...
-
As of this morning, a firm had a ledger balance of $1,252 with no outstanding deposits or checks. Today, the firm deposited three checks in the amount of $63 each and wrote a check in the amount of...
-
How would the cyclic voltammetry shown in Fig. 8.53a differ if (a) The Os(IV) complex decomposed rapidly; (b) Os(III) is oxidized in a single, rapid two-electron step to Os(V)? Figure 8.53a. i/mA 10...
-
The solution 31 P-NMR spectrum of P 4 S 3 consists of a doublet and quartet with intensities in the ratio 3:1. Suggest a structure consistent with this pattern.
-
Whats the most common cause of mass wasting events?
-
Your introduction needs to include the following. o Include a clear definition of unemployment and inflation and how and why they occur and rise in the economy. o Briefly provide your understanding...
-
Questions: 1. What strategies can be employed to foster a sense of inclusion and belonging within teams, and what are the potential benefits of doing so? 2. How can a team be successful? 3. What is...
-
Critical reflection involves closely examining events and experiences from different perspectives to inform future practice. In a few paragraphs, explain - Why educators should regularly reflect on...
-
What resources does the school or school district provide to teachers to promote diversity, equity, and inclusion? What are some of the strengths and shortcomings of the school's policies on...
-
Select FOUR companies listed on the UK Stock Exchange. Chose two companies from one industry sector and two other companies from another industry sector. By using the most recent three years'...
-
Refer to the Business and Society (March 2011) study on the sustainability behaviors of CPA corporations, Exercise 1.28 (p. 27). Corporate sustainability, recall, refers to business practices...
-
The following information is for Montreal Gloves Inc. for the year 2020: Manufacturing costs Number of gloves manufactured Beginning inventory $ 3,016,700 311,000 pairs 0 pairs Sales in 2020 were...
-
Comment on the modes of bonding of the ligands in the Mn(II) complexes listed at the end of Section 21.8, drawing attention to any conformational restrictions. Data from Section 21.8 Pr Pr iPr Mn iPr...
-
Values of oct for [Ni(OH 2 ) 6 ] 2+ and high-spin [Mn(OH 2 ) 6 ] 3+ have been evaluated spectroscopically as 8500 and 21000 cm 1 respectively. Assuming that these values also hold for the...
-
For which of the following ions would you expect the spin-only formula to give reasonable estimates of the magnetic moment: (a) [Cr(NH 3 ) 6 ] 3+ , (b) [V(OH 2 ) 6 ] 3+ , (c) [CoF 6 ] 3 ? Rationalize...
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...

Study smarter with the SolutionInn App


