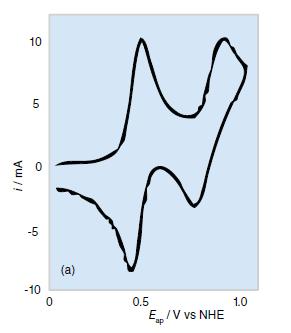
How would the cyclic voltammetry shown in Fig. 8.53a differ if (a) The Os(IV) complex decomposed rapidly;
Question:
How would the cyclic voltammetry shown in Fig. 8.53a differ if
(a) The Os(IV) complex decomposed rapidly;
(b) Os(III) is oxidized in a single, rapid two-electron step to Os(V)?
Figure 8.53a.
Transcribed Image Text:
i/mA 10 LO 5 O -5 -10 0 (a) 0.5 Ep/V vs NHE ap 1.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
a If the OsIV complex decomposed rapidly the second wave in the cyclic voltam...View the full answer

Answered By

RASEENA CK
computer science degree completed at calicut university an done yeardegree of library science at ignou university.I am worked in private education centre by tutoring students miainly my subject is mathematics.and i conducted other subject like computr physics
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
To carefully prepared mitochondria were added succinate, oxidized cytochrome c, ADP, orthophosphate, and sodium cyanide. Referring to Figure 14.16, answer the following. (a) List the sequence of...
-
Is an isothermal process necessarily internally reversible? Explain your answer with an example.
-
Presented below is information related to Alexis and Ryans, Attorneys at Law. Retained earnings, January 1, 2015 ......$ 23,000 Legal service revenue2015 ......... 340,000 Total expenses2015...
-
Stitch Fix is an online personal styling service that was founded in 2011 by entrepreneur Katrina Lake. Lake's vision was to disrupt the fashion retail industry by curating personalized wardrobe...
-
Calculate the weighted average cost of capital for a business and assess its usefulness when making investment decisions.
-
Which method for preparing the Operating Activities section of the statement of cash flows, the direct or the indirect method, do you believe provides more information to users of the statement?...
-
Black Media Inc. owns and operates a large number of newspapers across Canada. On October 1 20X5, the board of directors voted unanimously to dispose of one of those newspaper, The Daily Con. Black...
-
Sketch the forms of the following solution phase NMR spectra (abundances shown as per cent): (a) The 1 H-NMR spectrum of KBH 4 (b) The 1 H- and 195 Pt-NMR spectra of cis-[Pt(CO) 2 (H)Cl] 1 H, I = ,...
-
Determine the g values of the EPR spectrum shown in Fig. 8.57, measured for a frozen sample using a microwave frequency of 9.43 GHz. Figure 8.57 300 340 380 420 B/mT 460 500
-
A voltage source \(\mathrm{V}_{\mathrm{S}}=100 \angle 90^{\circ} \mathrm{V}\) is connected in series to a resistor of \(10 \Omega\) and an inductor of \(j 10 \Omega\). Find the phasor current...
-
1. Make a comparison between the leadership approaches "Trait Models" and "Behavioral Models". Discuss the main postulates and differences between the models and provide examples of a theory...
-
Applying the Central Limit Theorem: The amount of contaminants that are allowed in food products is determined by the FDA ( Food and Drug Administration ) . Common contaminants in cow milk include...
-
A collection of techniques used by social scientists to compile, summarize, and convey numerical data. Revised Research Question Hypothesis : Null Hypothesis : A null hypothesis, often known as H0,...
-
Adidas is an international sporting apparel/shoes brand. If Adidas was to enter a new foreign market, it would conduct a country market assessment. Identify the 4 components of the assessment....
-
Using the scenario linked in the Supporting Materials section, assume that you are the cost accountant for your company, and the CFO has asked for your analysis on purchasing materials from an online...
-
Refer to the International Journal of Statistical Distributions (Vol. 1, 2015) study of a variable life insurance policy, Exercise 4.97 (p. 238). Recall that a ratio (x) of the rates of return on the...
-
Assume a simple Keynesian depression economy with a multiplier of 4 and an initial equilibrium income of $3,000. Saving and investment equal $400, and assume full employment income is $4,000. a. What...
-
How would you attempt to (a) Estimate the crystal field stabilization energy of FeF 2 , (b) Determine the overall stability constant of [Co(NH 3 ) 6 ] 3+ in aqueous solution given that the overall...
-
Suggest the formula and structure of the mononuclear complex formed between Cr 3+ and ligand 21.83. Comment on possible isomerism. CO N CO N (21.83) CO
-
(a) Which of the following complexes would you expect to suffer from a JahnTeller distortion: [CrI 6 ] 4 , [Cr(CN) 6 ] 4 , [CoF 6 ] 3 and [Mn(ox) 3 ] 3 ? Give reasons for your answers. (b) [Et 4 N] 2...
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid
-
uppose a taxpayer has exhausted his lifetime exclusion amount and has $14 million. a. Assuming a flat 40% gift tax rate, what is the maximum amount a taxpayer can transfer to her daughter (and still...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...

Study smarter with the SolutionInn App


