How would you attempt to (a) Estimate the crystal field stabilization energy of FeF 2 , (b)
Question:
How would you attempt to
(a) Estimate the crystal field stabilization energy of FeF2,
(b) Determine the overall stability constant of [Co(NH3)6]3+ in aqueous solution given that the overall formation constant for [Co(NH3)6]2+ is 105, and:
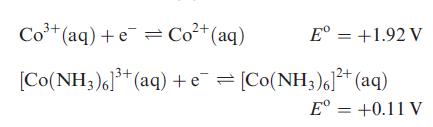
Transcribed Image Text:
Co+(aq)+e=Cot(aq) Eº = +1.92 V [Co(NH3)6]³+ (aq) + e¯ = [Co(NH3)6]²+ (aq) Eº = +0.11 V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
a Estimating the crystal field stabilization energy CFSE of FeF2 Crystal field stabilization energy is a term used in coordination chemistry to descri...View the full answer

Answered By

Surendar Kumaradevan
I have worked with both teachers and students to offer specialized help with everything from grammar and vocabulary to challenging problem-solving in a range of academic disciplines. For each student's specific needs, I can offer explanations, examples, and practice tasks that will help them better understand complex ideas and develop their skills.
I employ a range of techniques and resources in my engaged, interesting tutoring sessions to keep students motivated and on task. I have the tools necessary to offer students the support and direction they require in order to achieve, whether they need assistance with their homework, test preparation, or simply want to hone their skills in a particular subject area.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How would you attempt to assess whether the technology used by an industry in a developing country was inappropriate?
-
You have heard that the average American male is 69.7 inches tall. Yet almost all of the adult males in your personal world seem to be between 5 and 6 feet tall, with a few just over 6 feet. So how...
-
The recently completed new building to house the exhibits and staff of the Central City Museum was located adjacent to the campus of a private university. The new building was financed by the...
-
Mary, Ali and Nzioka are trading as Mali enterprises. They share profits and losses in the ratio of 2 = 1 1 respectively. The following is a statement of comprehensive income for the year ended 31st...
-
A construction project is broken down into the following 10 activities: a. Draw the network diagram. b. Find the critical path. c. If activities 1 and 10 cannot be shortened, but activities 2 and 9...
-
Innovate International is a large organization with few qualified suppliers. After conducting a portfolio analysis it was found that design and quality of the supplies are critical for Innovate...
-
Which of the following is not one of the FASBs initiatives to converge with IASB standards? LO4 a. The FASB eliminates differences between FASB and IASB standards by adopting IASB require ments, or...
-
Sheldon Optics produces medical lasers for use in hospitals. The accounts and their balances appear in the ledger of Sheldon Optics on October 31 of the current year as follows: Preferred 2% Stock,...
-
1-Sep Kawabata begins practice as a dentist, invests $20,000 cash 2-Sep Purchases dental equipment on account from Green Jacket Co. for $17,280 4-Sep Pays rent for office space, $680 for the month...
-
Figure 21.44 shows the change in concentration of [MnO 4 ] with time during a reaction with acidified oxalate ions. (a) Suggest a method of monitoring the reaction. (b) Explain the shape of the...
-
Suggest the formula and structure of the mononuclear complex formed between Cr 3+ and ligand 21.83. Comment on possible isomerism. CO N CO N (21.83) CO
-
Let L: U V be a linear function between inner product spaces. Prove that u R n solves the inhomogeneous linear system L[u] = f if and only if Explain why Exercise 3.1.11 is a special case of this...
-
1. [-/1 Points] DETAILS Find the associated exponential decay or growth model. (Round all coefficients to three significant digits.) Q=3,000 when t = 0; doubling time = 4 Q = Need Help? Read It Watch...
-
The 10 m wide gate restrains water at a depth of 6 m. Calculate the magnitude of the hinge reaction at A, and the contact force between the gate and the smooth surface B. Neglect the weight of the...
-
Write a program loop, using a pointer and a counter, that dears i'o 0 the contents of hexadecimal locations 500 through 5FF. Write a program to multiply two positive numbers by a repeated addition...
-
entries. Record journal entries in the order presented in the problem. If no entry is required, select "No Entry" for the account titles and enter O for the amounts. Credit account titles are...
-
APA formatting : Take the information below and put it into correct APA format. Journal Article (5 points) Authors: William R. Johnson, Cynthia E. Hernandez, Jamal M. Wilson. Year published: 2019....
-
On January 1, 2019, Stern Corporation purchased 100 shares of common stock issued by Milstein Inc. (representing 12% of the total shares outstanding) for $6,000 and 500 shares of Heifetz Inc....
-
Complete the equations for the following equilibria and calculate Keq where the Keq expression includes [HO]. Be sure to enter Keq in proper scientific notation. (a) ammonia (acting as a base) reacts...
-
Predict the product(s) for each of the following reactions. In each case, make sure to consider the number of chirality centers being formed. a. b. c. d. e. f. Os0, (catalytic) NMO 1) OsO, 2) NaHSO,...
-
Draw the propagation steps that achieve the autooxidation of diethyl ether to form a hydroperoxide: OOH A hydroperoxide Diethyl ether
-
Myo-Inositol is a polyol (a compound containing many OH groups) that serves as the structural basis for a number of secondary messengers in eukaryotic cells. Draw the more stable chair conformation...
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


