Discuss the bonding between the central p-block elements in the following compounds and give the expected arrangements
Question:
Discuss the bonding between the central p-block elements in the following compounds and give the expected arrangements of the organic substituents with respect to the central E2-unit:
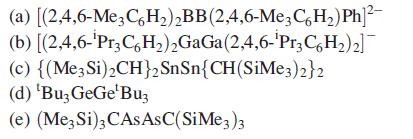
Transcribed Image Text:
(a) [(2,4,6-Me3C6H₂)₂BB (2,4,6-Me3C6H₂) Ph]²- (b) [(2,4,6-'Pr3C6H₂)2GaGa (2,4,6-'Pr3C6H₂)2] (c) {(Me3 Si)₂CH}2 (d) 'Bu3 GeGe¹Buz (e) (Me3 Si)3 CASASC(SiMe3)3 SnSn{CH(SiMe3)2}2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
To discuss the bonding between the central pblock elements in the given compounds we need to identify the central elements E and their coordination en...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
The NMR spectra below are for the organic compounds C6H12 and C4H10O. Deduce the structures for these compounds. See Exercise 70 for a discussion of the bonding in organic compounds. The structure of...
-
Two franchising experts recently debated the issue of whether new college graduates should consider franchising as a pathway to entrepreneurship. Jeff Elgin said recent college graduates are not...
-
Suppose Columbia Sportswear Company had accounts receivable of $299,585,000 at January 1, 2014, and $226,548,000 at December 31, 2014. Assume sales revenue was $1,244,023,000 for the year 2014. What...
-
During a Gemba Walk, what are some of the questions that a good manager should be asking those he/she might encounter? Defend the question you would pose (or the Gemba Manager would pose), with...
-
Briefly profile the clusters for the winning model from the previous exercise.
-
Described below are potential financial statement misstatements that are encountered by auditors in the audit of inventory and cost of goods sold. a. Management of a chain of discount department...
-
On October 31, 2015, Zeyn sold the property whose its cost of goods was 60,000 at a price of $75,000. he received a down payment of $20,000 remaining monthly installments with the first installment...
-
Whether the bonding in lithium alkyls is predominantly ionic or covalent is still a matter for debate. Assuming a covalent model, use a hybrid orbital approach to suggest a bonding scheme for (MeLi)...
-
Write down formulae for the following ions: (a) Manganate(VII); (b) Manganate(VI); (c) Dichromate(VI); (d) Vanadyl; (e) Vanadate (ortho and meta); (f) Hexacyanidoferrate(III). Give an alternative...
-
A piston cylinder has R-134a at 20oC, 100 kPa which is compressed to 500 kPa in a reversible adiabatic process. Find the final temperature and the specific work.
-
For this online discussion, we will explore the relevance of various management styles in the context of your respective organizations. Your task is to review different management styles and propose...
-
Is a t-Distribution Appropriate? A sample with size n = 10 has x = 508.5, and s = 21.5. The dotplot for this sample is given below. 0000 00 500 510 520 530 540 550 560 570 Indicate whether or not it...
-
Interpret the results. Write a statement to summarize your conclusion. Is a relationship present? Do we accept or reject the null hypothesis? Are the two variables related? Why or why not?
-
Case study information Australian Renewable Energy Hub Source: https://research.csiro.au/hyresource/australian-renewable-energy-hub/ April 20th, 2023 The Australian Renewable Energy Hub (AREH) will...
-
Listening is a crucial leadership skill that is essential for building effective relationships and solving problems. Write a paper that explores the importance of listening as a leadership skill,...
-
Cagney Company sold $200,000 of bonds on July 1, 2018. A portion of the amortization table appears below. Required: 1. Indicate the stated interest rate on these bonds. 2. Calculate the effective...
-
Refer to the Conservation Ecology (Dec. 2003) study of the causes of forest fragmentation, presented in Exercise 2.166 (p. 97). Recall that the researchers used advanced high-resolution satellite...
-
You get a PT phase diagram by projecting a PVT phase diagram on the PT plane.
-
The mechanism of the following transformation involves a carbocation intermediate that rearranges in a way that we have not yet seen. Rather than occurring via a methyl shift or a hydride shift, a...
-
An ideal gas is expanded adiabatically into a vacuum. Decide which of q, w, U and H is positive, negative, or zero.
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


