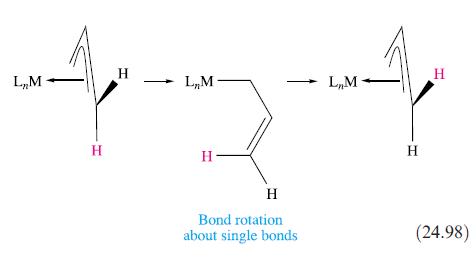
Explain why scheme 24.98 is invoked to explain the equivalence of the H atoms in each terminal
Question:
Explain why scheme 24.98 is invoked to explain the equivalence of the H atoms in each terminal CH2 group of an η3-allyl ligand, rather than a process involving rotation about the metal–ligand coordination axis.
Transcribed Image Text:
L,M H H L₁M H- H Bond rotation about single bonds L₁M H H (24.98)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
Scheme 2498 is invoked to explain the equivalence of the H atoms in each terminal CH2 group of an 3a...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
(a) Confirm that H 2 Os 3 (CO) 11 has sufficient valence electrons to adopt a triangular metal framework. Do the modes of bonding of the CO and H ligands affect the total valence electron count?...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Zebra Corporation has always been an S corporation and is 100% owned by Paul. Paul has a basis of $40,000 in his Zebra stock at the beginning of the year. During the year, Zebra has an ordinary loss...
-
If Neer Company had net income of $300,000 in 2013 and it experienced a 24.5% increase in net income for 2014, what is its net income for 2014? If 6 cents of every dollar of Neers revenue is net...
-
Find a vector field with twice-differentiable components whose curl is xi + yj + zk or prove that no such field exists.
-
Should these charts be carried out on the training data set or the test data set? Why?
-
On December 31, Year 1, Precision Manufacturing Inc. (PMI) of Edmonton purchased 100% of the outstanding ordinary shares of Sandora Corp. of Flint, Michigan. Sandora's comparative statement of...
-
Current portion of long - term debt Connie's Bistro, Inc. reported the following information about its long - term debt in the notes to a recent financial statement ( in millions ) : Long - term debt...
-
Reaction of Fe(CO) 5 with Na 2 [Fe(CO) 4 ] in THF gives a salt Na 2 [A] and CO. The Raman spectrum of [Et 4 N] 2 [A] shows an absorption at 160 cm 1 assigned to an unbridged FeFe bond. Suggest an...
-
The reaction of 1,3-dimethylimidazolium iodide (shown on the next page) with one equivalent of KO t Bu in THF, followed by addition of one equivalent of Ru 3 (CO) 12 leads to product A. The IR...
-
(a) Let c F, where F is a field of characteristic p (p prime). Then x P - x - c is irreducible in F[x] if and only if x p - x - c has no root in F. (b) If char F = 0, part (a) is false.
-
Finding Confidence Intervals. In Exercises 9-16, assume that each sample is a simple random sample obtained from a population with a normal distribution. Professor Evaluation Scores Listed below are...
-
Pacifico Company, a U . S . - based importer of beer and wine, purchased 1 , 7 0 0 cases of Oktoberfest - style beer from a German supplier for 4 5 9 , 0 0 0 euros. Relevant U . S . dollar exchange...
-
7.C. a. When you add two vectors you get another vector: yes or no? b. When you subtract two vectors you get another vector: yes or no? c. Given the coordinate system below where increasing numbers...
-
Problem 1 At a given instant, the position of a plane at A and a train at B are measured relative to a radar antenna at O. Determine the distance d between A and B at this instant. To solve the...
-
The Bell-Boeing V-22 Osprey tiltrotor is both an airplane and a helicopter. It's advantage is the ability to rotate its engines and rotors to vertical position for take-off, landings, and helicopter...
-
Travis Corporation expected to pay its stockholders a dividend in January 2020. The cash dividend of $75,000 was declared on December 31, 2019. Required: What is the appropriate journal entry to...
-
I frequently use NY Times and CNN and am aware of Fox News but I never use it. I visit these sites, NY Times and CNN, a few times a week whenever I have to research something or see something on...
-
Predict whether each of the following compounds should be aromatic. a. b. c.
-
The cyclopropenyl cation has a three-membered ring that contains a continuous system of overlapping p orbitals. This system contains a total of two Ï electrons. Using a Frost circle, draw an...
-
Arrange each set of isomeric alkenes in order of stability. a. b.
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


