For each of the following complexes, give the oxidation state of the metal and its d n
Question:
For each of the following complexes, give the oxidation state of the metal and its dn configuration:
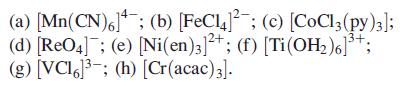
Transcribed Image Text:
(a) [Mn(CN)6]; (b) [FeC¹4]¯¯; (c) [CoCl3 (py)3]; (d) [ReO4]; (e) [Ni(en)3]2+; (f) [Ti (OH₂)6]³+; (g) [VCI6³; (h) [Cr(acac) 3].
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
To determine the oxidation state of the metal and its d electron configuration in each complex we need to apply some general principles of coordinatio...View the full answer

Answered By

Akash M Rathod
I have been utilized by educators and students alike to provide individualized assistance with everything from grammar and vocabulary to complex problem-solving in various academic subjects. I can provide explanations, examples, and practice exercises tailored to each student's individual needs, helping them to grasp difficult concepts and improve their skills.
My tutoring sessions are interactive and engaging, utilizing a variety of tools and resources to keep learners motivated and focused. Whether a student needs help with homework, test preparation, or simply wants to improve their skills in a particular subject area, I am equipped to provide the support and guidance they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
You have decided to purchase additional memory for your computer in order to better support the latest version of the Windows operating system. At the local computer store, you notice that not only...
-
Give the number of (valence) d electrons associated with the central metal ion in each of the following complexes (a) K3[Fe(CN)6], (b) [Mn(H2O)6](NO3)2, (c) Na[Ag(CN)2], (d) [Cr(NH3)4Br2]ClO4, (e)...
-
Give the outer electron configuration for each of the following columns in the periodic table. 1) 3A Express your answer as a string without blank space between orbitals. For example, the outer...
-
A taxpayer had the following income: a.) Gain on sale of domestic stocks - P200,000............ b.) Gain on sale of foreign bonds - P100,000............ c.) Gain on sale of a commercial lot in...
-
Order the following major concepts that have helped define the OSCM field on a time line. Use 1 for the earliest to be introduced, and 5 for the most recent. _______Supply chain management...
-
Find the mass and first moments about the coordinate axes of a thin square plate bounded by the lines x = 1, y = 1 in the xy-plane if the density is (x, y) = x 2 + y 2 + 1/3.
-
C12.14. Cana firmhavea highPIE ratioyeta lowP/Bratio? Howwould youcharacterize the growth expectations forthis firm?
-
Selected accounts for Brianna??s Salon are presented below. All June 30 postings are from closing entries.Instructions(a) Prepare the closing entries that were made.(b) Post the closing entries to...
-
Question 2 (mark-to-market) You enter a long position in a future contract with the size of 125,000 today. The futures expire in 90 days. The interest rates are i$=5% and ie=10.4%. The current spot...
-
The vibrational modes of KrF 2 are at 590, 449 and 233 cm 1 . Explain why only the bands at 590 and 233 cm 1 are observed in the IR spectrum of gaseous KrF 2 .
-
By referring to the following literature source, assess the safety precautions required when handling XeO 4 : M. Gerken and G.J. Schrobilgen (2002) Inorg. Chem., vol. 41, p. 198.
-
How does charity care affect the patient service revenue reported by hospitals, and what measure of charity care are hospitals required to disclose in the notes to their financial statements?
-
2 4 . In the current year, Madison sold Section 1 2 4 5 property for $ 6 , 0 0 0 . The property cost $ 2 6 , 0 0 0 when it was purchased 5 years ago. The depreciation claimed on the property was $ 2...
-
Swifty Company purchased machinery on January 1, 2025, for $82,400. The machinery is estimated to have a salvage value of $8,240 after a useful life of 8 years. (a) Your answer is incorrect. Compute...
-
Currently, the unit selling price is $ 5 0 , the variable cost is $ 3 4 , and the total fixed costs are $ 1 0 8 , 0 0 0 . a . Compute the current break - even sales in units.
-
(1) The Mean Value Theorem states: Let f be continuous over the closed [a, b] and differentiable over the open interval (a, b). Then, there exists at least one point c E (a, b) such that: f(b) - f(a)...
-
Assume you are an Israeli investor; the symbol for the Israeli currency, the shekel, is ILS. You see that stock for Top Image has a bid price of ILS 17 and an ask price of ILS 19 in Israel, a bid...
-
The income statement and comparative balance sheet for Front Row Entertainment are shown below: Front Row Entertainment Inc. Income Statement For the year ended December 31, 2020 Revenues: Sales...
-
You are planning to purchase your first home five years from today. The required down payment will be $50,000. You currently have $20,000. but you plan to contribute $500 each quarter to a special...
-
As explained previously, the concentration of an alcohol can be selected such that both a broad signal and a narrow signal appear simultaneously. In such cases, the broad signal is always to the...
-
For each of the following IR spectra, identify whether it is consistent with the structure of an alcohol, a carboxylic acid, or neither. a. b. c. d. e. f. 100- 80- 60- 40 20- 0. 2000 4000 3500 3000...
-
For each of the following IR spectra, determine whether it is consistent with the structure of a ketone, an alcohol, a carboxylic acid, a primary amine, or a secondary amine. a. b. c. d. e. f. 100-...
-
You are the digital marketing director for High West fashions, a regional clothing company that specializes in custom t-shirts. Your company has decided to launch an online advertising campaign that...
-
In-the-money put options will automatically get exercised at the expiration. True OR False
-
Which of the following examples of business-use property is NOT eligible for Section 1231 treatment when sold at a gain? * Sale of land held for three years. Net gain from a casualty gain on a dump...

Study smarter with the SolutionInn App


