Four of the lines in the Balmer series are at 656.28, 486.13, 434.05 and 410.17 nm. Show
Question:
Four of the lines in the Balmer series are at 656.28, 486.13, 434.05 and 410.17 nm. Show that these wavelengths are consistent with eq. 1.4.
Data from Equation 1.4.
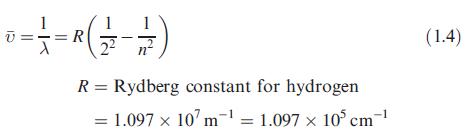
Transcribed Image Text:
1 0 = = = R ( 2²/2 - 1/2 ) R = Rydberg constant for hydrogen = 1.097 x 107 m¹ = 1.097 x 105 cm-¹ 10³ (1.4)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
To show that the given wavelengths are consistent with Equation 14 we need to use the Rydberg formul...View the full answer

Answered By

User l_917591
As a Business Management graduate from Moi University, I had the opportunity to work as a tutor for undergraduate students in the same field. This experience allowed me to apply the theoretical knowledge I had gained in a practical setting, while also honing my teaching and communication skills.
As a tutor, I was responsible for conducting tutorial sessions, grading assignments and exams, and providing feedback and support to my students. I also assisted with the preparation of course materials and collaborated with other tutors and professors to ensure consistency in teaching and assessment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
The Bohr model can be applied to singly ionized helium He+ (Z = 2). Using this model, consider the series of lines that is produced when the electron makes a transition from higher energy levels into...
-
A company had a broken printer which they deemed was not worth fixing and thus discarded it. The original cost of the printer was $7,500 and the accumulated depreciation at the time of disposal was...
-
Doreen Dunst started an interior design company called Interiors by Doreen, Inc., on June 1, 2012. The following amounts summarize the financial position of her business on June 14, 2012, after the...
-
In Exercises explain why the evaluation of the integral is incorrect. Use the integration capabilities of a graphing utility to attempt to evaluate the integral. Determine whether the utility gives...
-
4. In connection with Thurman Townships long-term debt, the following cash accumulations are available to cover payment of principal and interest on: Bonds for financing of water treatment plant...
-
The balance sheet of Lamont Bros. follows: a. What portions of Lamonts assets were provided by debt, contributed capital, and earned capital? Reduce contributed capital by the cost of the treasury...
-
D Question 1 Explain how the income tax system is an automatic stabilizer for the tax payer. (20-100 words)
-
Using data from Appendix 8, construct a graph to show the trend in the third ionization energies of the elements from Li to Kr. Compare the graph with that shown in Fig. 1.16, and rationalize what...
-
Chromium has four isotopes, How many electrons, protons and neutrons does each isotope possess? 50 54 Cr, Cr, Cr and Cr. 53 54 24 24
-
1. Search the Web for functional organizational structures. Summarize at least one website and compare it to what was presented in this chapter. What new insights did you gain from this website?
-
1. Why do companies that choose to open subsidiaries in other countries have different HR responsibilities? 2. How has globalization allowed companies to become "global companies" more easily? 3....
-
Is Kroger's innovation Product-related or process-related? Do the innovations tend to be incremental or radical? https://www.thekrogerco.com/about-kroger/our-business/ Kroger Co. opens new spoke in...
-
Define what is Process Mapping/Value Stream Mapping How do you apply process mapping methodology? What are the advantages of leaders using process mapping Identify a real world business...
-
What role do formalized processes and protocols play in highly structured organizations, and how can organizations balance the need for structure with the imperative for flexibility and innovation ?
-
In what ways do decision-makers balance quantitative data with qualitative insights to optimize complex strategic choices, especially in high-stakes business environments where traditional metrics...
-
(a) Predict the mean wind speed of all hurricanes whose atmospheric pressure is 950 mb. (b) Construct a 95% confidence interval for the mean wind speed found in part (a). (c) Predict the wind speed...
-
Troy is a qualified radiologist who operates a successful radiology practice from purpose- built rooms attached to his house. Troy works in the practice three days a week, and the other two days he...
-
Identify the products from the reaction between the following pairs of reagents. In each case identify the species which are acting as a Lewis acid or a Lewis base in the reactions. (a) CsF + BrF 3...
-
Use the data in Table 5.5 to calculate the enthalpy change for the reaction of iodine with phenol. Table 5.5. TABLE 5.5 Drago-Wayland parameters for some acids and bases* E Acids Antimony...
-
For each of the following processes, identify the acids and bases involved and characterize the process as complex formation or acidbase displacement. Identify the species that exhibit Brnsted...
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


