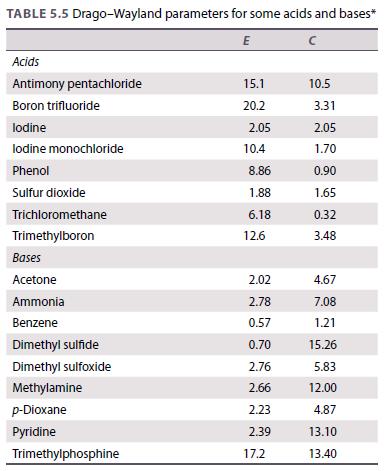
Use the data in Table 5.5 to calculate the enthalpy change for the reaction of iodine with
Question:
Use the data in Table 5.5 to calculate the enthalpy change for the reaction of iodine with phenol.
Table 5.5.
Transcribed Image Text:
TABLE 5.5 Drago-Wayland parameters for some acids and bases* E Acids Antimony pentachloride Boron trifluoride lodine lodine monochloride Phenol Sulfur dioxide Trichloromethane Trimethylboron Bases Acetone Ammonia Benzene Dimethyl sulfide Dimethyl sulfoxide Methylamine p-Dioxane Pyridine Trimethylphosphine 15.1 20.2 2.05 10.4 8.86 1.88 6.18 12.6 2.02 2.78 0.57 0.70 2.76 2.66 2.23 2.39 17.2 10.5 3.31 2.05 1.70 0.90 1.65 0.32 3.48 4.67 7.08 1.21 15.26 5.83 12.00 4.87 13.10 13.40
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
To calculate the enthalpy change for the reaction of iodine with phenol we can use the st...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
In the following exercises, you will use the data in the TAL Distributors database shown in Figure 2-1 in Chapter 2. (If you use a computer to complete these exercises, use a copy of the original TAL...
-
In the following exercises, you will use the data in the Solmaris Condominium Group database shown in Figures 1-21 through 1-25. (If you use a computer to complete these exercises, use a copy of the...
-
In the following exercises, you will use the data in the Solmaris Condominium Group database shown in Figures 1-21 through 1-25 in Chapter 1. (If you use a computer to complete these exercises, use a...
-
Three disease-carrying organisms decay exponentially in lake water according to the following model: Estimate the initial population of each organism (A, B, and C) given the followingmeasurements:...
-
Amner Manufacturing Company had an excellent year. The company hired a new marketing director in January. The new directors great motivational appeal inspired the sales staff, and, as a result, sales...
-
What concerns did suppliers and designers have? How did Rent the Runway's founders dispel these concerns? Describe how the firm seeks to build deep and mutually beneficial industry partnerships.
-
Shriman operates a taxi. Compute cost per running km from the following details. Rs Purchase price of taxi 50,000 Insurance per annum 1,000 Rent of garage per month 100 Tyres and tubes per set (A set...
-
James Carpenter contracted with Austin Estates, LP, to buy property in Texas. Carpenter asked Sandra McBeth to invest in the deal. He admitted that a dispute had arisen with the city of Austin over...
-
assume that a project has the following cash flows: -200/35/45/55/65/75. The IRR of this project will be (enter as a raw number)
-
Identify the products from the reaction between the following pairs of reagents. In each case identify the species which are acting as a Lewis acid or a Lewis base in the reactions. (a) CsF + BrF 3...
-
For each of the following processes, identify the acids and bases involved and characterize the process as complex formation or acidbase displacement. Identify the species that exhibit Brnsted...
-
Why did baby boomers have such an important impact on consumer culture in the second half of the twentieth century?
-
Locate a scholarly article relevant to how to present your financial plan for opening a Roller Skating Rink (from your draft business plan) to a lending institution--and describe your strategy for...
-
How would you expect seasonal fluctuations in demand to affect a rental company's decisions about pricing rented products such as wedding dresses or convertible cars? In terms of pricing principles,...
-
Do we drive technology, or does technology drive us? If technology drives us, what are the risks? The other side of the coin would be that we are able to stay ahead of technological transformations....
-
How do you explain the differences between the two analyses and what are the implications of using the BCG matrix in practice?
-
How do leadership styles, such as transformational leadership, shared leadership, and servant leadership, impact team dynamics, member motivation, and overall team effectiveness ?
-
Watch the videos, Fibre (Fiber) vs Copper as Fast as Possible and Tri Band WiFi as Fast as Possible, discuss at least two concepts you learned from the videos and provide rationale of your response.
-
In the synthesis of the keto acid just given, the dicarboxylic acid decarboxylates in a specific way; it gives Explain. HO rather than HO
-
Using data from Appendix 11, and the value for the standard Gibbs energy of formation for PbS of 99 kJ mol 1 , determine a value for K sp for this salt. Data from Appendix 11 The concentration of...
-
In hydrochloric acid, HOI reacts to give [ICl 2 ] . Use the potential diagrams below to explain why HOI disproportionates in aqueous acidic solution, but does not when the acid is aqueous HCl. [03]...
-
(a) Using the potential diagram below (at pH 14), calculate E O3 2 /O 2 (b) Comment on the following data: (c) How valid is Fig. 8.4a for aqueous solutions at pH 2? Figure 8.4a. 03 +0.66 03- +1.25 E 0
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


