In hydrochloric acid, HOI reacts to give [ICl 2 ] . Use the potential diagrams below
Question:
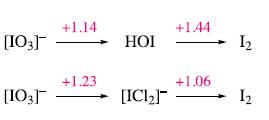
In hydrochloric acid, HOI reacts to give [ICl2]−. Use the potential diagrams below to explain why HOI disproportionates in aqueous acidic solution, but does not when the acid is aqueous HCl.
Transcribed Image Text:
[03] [03] +1.14 +1.23 НОЇ [ICI2] +1.44 +1.06 Iz Iz
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To understand why HOI disproportionates in aqueous acidic solution but not in aqueous HCl we need to ...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In a laboratory, two liquids, A and B, were found in a box labeled only "isomeric alkyl halides C5H11Br." You have been employed to deduce the structures of these compounds from the following data...
-
When an acid reacts with a base: 1) This is a neutralization reaction 2) Pink color will appear in the resulting solution 3) Both of the answers are correct 4) None of the answers is correct QUESTION...
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
Assume that your team has been in contract with the headquarters of a company that owns several restaurants in different states in the US. Your team is to provide software that manages these...
-
Describe the three most common motives for acquisition.
-
Find the acute angle , to the nearest tenth of a degree, for the given function value. cot = 1.351
-
What do the four quadrants of the portfolio analysis represent?
-
Santo Corporation experienced a fire on December 31, 2014 in which its financial records were partially destroyed. It has been able to salvage some of the records and has ascertained the following...
-
Mon Inc. is a large retailer of shoes. An income statement for the company's most recent quarter for their top selling brand is presented below: Sales $200,000 Less cost of goods sold 132,000 Gross...
-
Using data from Appendix 11, and the value for the standard Gibbs energy of formation for PbS of 99 kJ mol 1 , determine a value for K sp for this salt. Data from Appendix 11 The concentration of...
-
(a) Using the potential diagram below (at pH 14), calculate E O3 2 /O 2 (b) Comment on the following data: (c) How valid is Fig. 8.4a for aqueous solutions at pH 2? Figure 8.4a. 03 +0.66 03- +1.25 E 0
-
Solve the equation x 2 + 7x = -6 by factoring.
-
Lazlo s estimates uncollectible accounts to be 0 . 9 % of sales. Its year - end unadjusted trial balance shows Accounts Receivable of $ 1 1 2 , 5 0 0 and sales of $ 9 6 5 , 0 0 0 . If Lazlo s uses...
-
Identify one or two of the best and one or two of the worst work teams on which you served as a member. 1. Identify the top three to five factors that made the team the best or the worst in terms of...
-
ColorCoder is a HousePaint Shop which supplies currently two types of house paints, namely, alpha and beta house paints. The shop is planning to sell a primer (paint base) and the needed paint...
-
which department adds value to a product or service that is observable by a customer?
-
Using Figure 14.1, answer the following questions: a. What was the settle price for July 2022 coffee futures on this date? What is the total dollar value of this contract at the close of trading for...
-
According to the U.S. Census Bureau, 7.1% of all babies born are of low birth weight ( <5 lb, 8 oz). An obstetrician wanted to know whether mothers between the ages of 35 and 39 years give birth to a...
-
Multiple Choice Questions: 1. The largest component of aggregate demand is? a. Government purchases. b. Net exports. c. Consumption. d. Investment. 2. A reduction in personal income taxes, other...
-
The increased reactivity that allows the design and synthesis of materials based on molecular units also means that these compounds are unsuitable for many applications that currently use inorganic...
-
Classify the oxides (a) BeO, (b) TiO 2 , (c) La 2 O 3 , (d) B 2 O 3 , (e) GeO 2 into glass-forming and non-glass-forming.
-
Compare and contrast the chemistries of graphite and C 60 with respect to their compounds in association with the alkali metals.
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...
-
1. Determine the value of the right to use asset and lease liability at commencement of the lease.
-
Problem 22-1 The management of Sunland Instrument Company had concluded, with the concurrence of its independent auditors, that results of operations would be more fairly presented if Sunland changed...

Study smarter with the SolutionInn App


