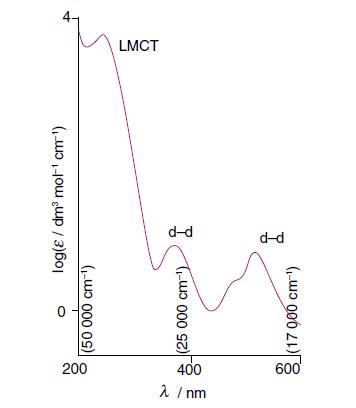
From the spectrum of [CrCl(NH 3 ) 5 ] 2+ shown in Fig. 20.33, propose a wavelength
Question:
From the spectrum of [CrCl(NH3)5]2+ shown in Fig. 20.33, propose a wavelength for photoinitiation of reduction of Cr(III) to Cr(II) accompanied by oxidation of a ligand.
Figure 20.33.
Transcribed Image Text:
200 λ /nm 400 log(ε/ dm³ mot¹ cm-¹) (50 000 cm-¹) (25 000 cm-¹) (17 900 cm-¹) d-d LMCT
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
In the spectrum of CrClNH352 shown in Figure 2033 the absorption peak at approximately 520 nm corres...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-4. Ivan and Irene paid the following in 2012 (all by check or can otherwise be...
-
The pH of microscopic vesicles (compartments) in living cells can be estimated by infusing an indicator (HIn) into the compartment and measuring the quotient [In-]/[HIn] from the spectrum of the...
-
Zainab company has sold goods on credit RO 55,000 on 31st December 2020 and received RO 15,000 towards credit sales. The company had debit balance of RO 5500 and the balance in accounts receivable...
-
In its first month of operation, Franklin Company purchased 120 units of inventory for $6, then 200 units for $7, and finally 140 units for $8. At the end of the month, 180 units remained. Compute...
-
Define the Sortino and Omega ratios and discuss their value in assessing ETF performance.
-
Calculating Future Value. Krista Lee can purchase a service contract for all of her major appliances for $180 a year. If the appliances are expected to last for 10 years and she earns 5 percent on...
-
Instantaneous Power in a Standing Wave. From Eq. (15.21), the instantaneous rate at which a wave transmits energy along a string (instantaneous power) is Where F is the tension. (a) Evaluate f (x, t}...
-
(5 points) In 2020, Schwei Co. overstated ending inventory by $740,000 and understated depreciation by $592,000. In 2021, Schwei Co. understated ending inventory by $1,036,000 and overstated...
-
Does the fact that [Ni(CN) 5 ] 3 can be isolated help to explain why substitution reactions of [Ni(CN) 4 ] 2 are very rapid?
-
In the presence of catalytic amounts of [Pt(-P 2 O 5 H 2 ) 4 ] 4 (21) and light, 2-propanol produces H 2 and acetone (E.L. Harley, A.E. Stiegman, A. Vlcek, Jr, and H.B. Gray, J. Am. Chem. Soc., 1987,...
-
A companys net sales are $675,000, its cost of goods sold is $459,000, and its net income is $74,250. Its gross margin ratio equals a. 32%. b. 68%. c. 47%. d. 11%. e. 34%.
-
Use as many directional terms as possible to describe therelationshipbetween: a. the antecubital region and the poplitealregion b. the acromial region and the mentalregion c. the gluteal region and...
-
Average rate of return-cost savings Maui Fabricators Inc. is considering an investment in equipment that will replace direct labor. The equipment has a cost of $114,000 with a $10,000 residual value...
-
Alan was rated as excellent on his individual work performance evaluation, earning him $2,000, provided as a merit pay increase. His annual salary this year is $48,000. He works in a team of 3...
-
Josie spends $60 at the end of each month on cigarettes. If shestops smoking and invests the same amount in an investment planpaying 6% compounded monthly, how much will she have after fiveyears? 2...
-
Say we have a Boeing 747 whose longitudinal flight dynamics for a given flight condition may be approximated using the following state equation (uncontrolled motion; thus no need to consider the...
-
Calculate the ratio of price to expected earnings for River Cruises both before and after it borrows the $250,000. Why does the P /E ratio fall after the increase in leverage?
-
Determine whether the lines are parallel, perpendicular, or neither. 2x + 3y = -12, 2y - 3x = 8
-
Using the chain rule for differentiation, show that the isobaric expansion coefficient expressed in terms of density is given by = (1/)(p/T) P .
-
Draw a bond-line structure for each of the following compounds: (a) 2-Heptyne (b) 2,2-Dimethyl-4-octyne (c) 3,3-Diethylcyclodecyne
-
Predict the products for each of the following reactions: (a) (b) H2 Lindlar's catalyst :? H2 Pt -? Na NH, (1) ? Ni,B Pd Na NH3 (/)
-
ABC Corporation has an activity - based costing system with three activity cost pools - Machining, Setting Up , and Other. The company's overhead costs, which consist of equipment depreciation and...
-
Consolidated Balance Sheets - USD ( $ ) $ in Thousands Dec. 3 1 , 2 0 2 3 Dec. 3 1 , 2 0 2 2 Current assets: Cash and cash equivalents $ 9 8 , 5 0 0 $ 6 3 , 7 6 9 Restricted cash 2 , 5 3 2 Short -...
-
How does corporate governance contribute to investor confidence and stakeholder trust? Accounting

Study smarter with the SolutionInn App


