Predict the products for each of the following reactions: (a) (b) H2 Lindlar's catalyst :? H2 Pt
Question:
(a)
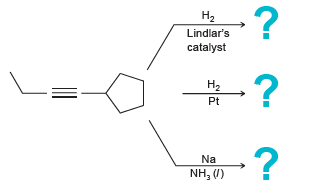
(b)
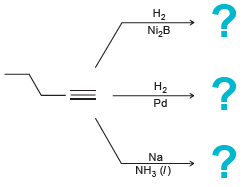
Transcribed Image Text:
H2 Lindlar's catalyst :? H2 Pt -? Na NH, (1) ? На Ni,B На Pd Na NH3 (/)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
a b H ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the products for each of the following reactions: a. b. c. d. . Ti[OCH(CH,),1. (+)-DET -- THCICH) (-)-DET
-
Predict the products for each of the following reactions, and propose a mechanism that explains the formation of each product: (a) (b) (c) (d) (e) (f) HCI HCI HCI
-
Predict the products for each of the following reactions, and in each case, determine which product will predominate: (a) (b) (c) HBr 40C HCI 0C
-
Draw with Ruler Touch Touch 4x For each of the three simple circuit boards you will need to calculate the total resistance, Req, for the entire circuit board by using the measured resistances of each...
-
The accounting records of Reynolds Corporation revealed the following selected costs: Sales commissions, $65,000; plant supervision, $190,000; and administrative expenses, $185,000. Reynolds's period...
-
A fire boat that travels 24.0 km/h in still water crosses a river to reach the opposite bank at a point directly opposite that from which it left. If the river flows at 5.0 km/h, what is the velocity...
-
Why can the end of a project be stressful for many of the project stakeholders? AppendixLO1
-
The cash flow statement categorizes like transactions for optimal reporting. Identify each of the following transactions as one of the following: Operating activity (O) Investing activity (I) ...
-
QUESTIOn 2 On January 1, 2021, David Mest Communications granted restricted stock units (RSUs) representing 30 million of its $1 par common shares to executives, subject to forfeiture if employment...
-
Katelyn's Catering ("Katelyn") provides food services for corporate events. In the month of December 2019, she was very busy with catering events and didn't have time to keep up with her accounting...
-
What are the four objectives described in this section?
-
Predict the products for each of the following reactions: H2 Lindlar's catalyst 2 Pt
-
Cost of goods sold using the FIFO is: a. $165,600. b. $150,000. c. $147,600. d. $122,400. e. None of the above.
-
how could playing in a sandbox help to the development of children? how could a garden help to the development of children? how could playground obstacle courses like a pebble bridge and monkey bars...
-
A store order bottles of shampoo throughout the year. Over time, the store has learned that the annual demand D for shampoo is constant, i.e., there is no variability. Currently, the store decides to...
-
Solve the Practice #2 == where L2 =02A = a, L404B = c, L = 0204 = d, y = /2 1) Find the velocity 3 when 82 = /2 and 6 = 0.4 rad/s 2) Find the acceleration 63 when = /2 and 62 = 0.4 rad/s 03. 03 Y B...
-
.0.5 0.5 For the above plot of the ellipsoid (22) 2- + +() + (-) = 1, find the parameters a, b and c. Note that a, b and c are positive integers between 1 and 6 inclusive. Use the mouse to rotate the...
-
The annual energy consumption of the University of Maryland is 100 million kWh. How much Uranium-235 is needed to produce this amount of energy in a nuclear power plant assuming 100% efficiency? (The...
-
In Problems 5170, find the domain of each function. f(x) -x -x-2
-
KD Insurance Company specializes in term life insurance contracts. Cash collection experience shows that 20 percent of billed premiums are collected in the month before they are due, 60 percent are...
-
Pentalene is a most elusive molecule and has never been isolated. The pentalene dianion, however, is well known and quite stable. Explain. 12- Pentalene Pentalene dianion
-
Indole is an aromatic heterocycle that has a benzene ring fused to a pyrrole ring. Draw an orbital picture of indole. (a) How many ? electrons does indole have? (b) What is the electronic...
-
Ribavirin, an antiviral agent used against hepatitis C and viral pneumonia, contains a 1, 2, 4-triazole ring. Why is the ringaromatic? 1,2,4-Triazole ring N- NH2 N-N Ribavirin OH
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...
Policy Reform And Chinese Markets Progress And Challenges 1st Edition - ISBN: 1847203965 - Free Book

Study smarter with the SolutionInn App


