Give an explanation for the following observations (part d assumes Box 29.2 has been studied): (a) Both
Question:
Give an explanation for the following observations (part d assumes Box 29.2 has been studied):
(a) Both haemoglobin and cytochromes contain haem-iron;
(b) Cytochrome c oxidase contains more than one metal centre;
(c) Each sub-unit in deoxyhaemoglobin contains 5-coordinate Fe(II), but in cytochrome c, the Fe centre is always 6-coordinate;
(d) Nitrophorin (NP1) reversibly binds NO.
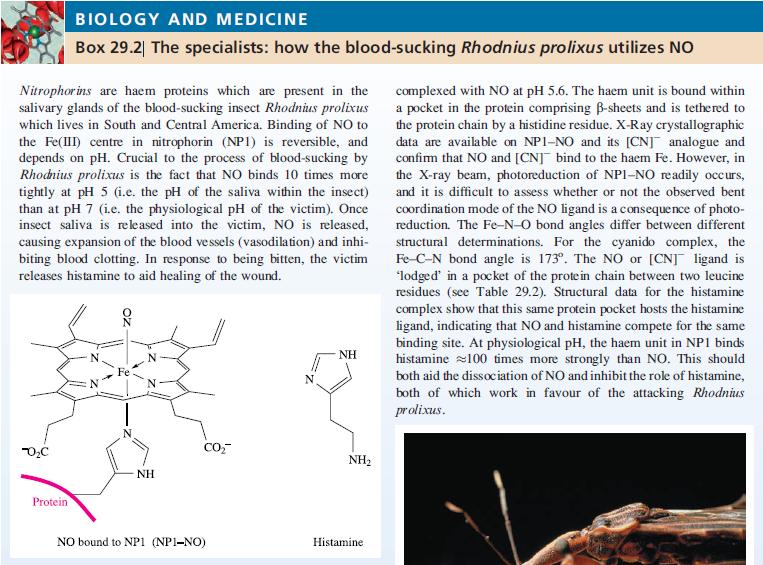
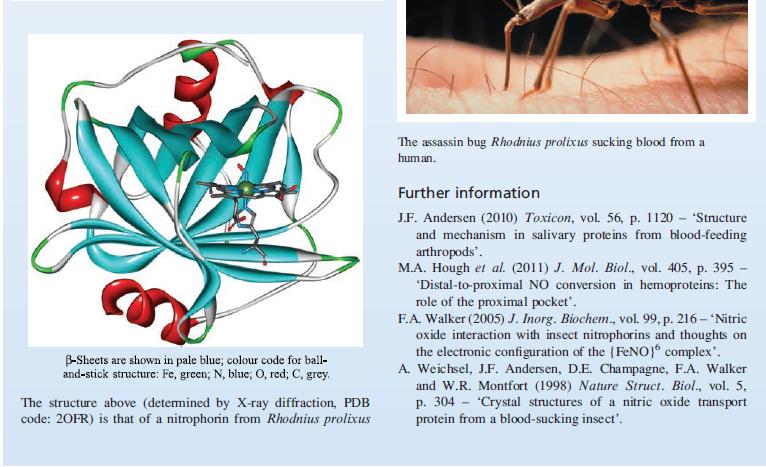
Transcribed Image Text:
Nitrophorins are haem proteins which are present in the salivary glands of the blood-sucking insect Rhodnius prolixus which lives in South and Central America. Binding of NO to the Fe(III) centre in nitrophorin (NPI) is reversible, and depends on pH. Crucial to the process of blood-sucking by Rhodnius prolixus is the fact that NO binds 10 times more tightly at pH 5 (i.e. the pH of the saliva within the insect) than at pH 7 (i.e. the physiological pH of the victim). Once insect saliva is released into the victim, NO is released, causing expansion of the blood vessels (vasodilation) and inhi- biting blood clotting. In response to being bitten, the victim releases histamine to aid healing of the wound. -0₂C BIOLOGY AND MEDICINE Box 29.2 The specialists: how the blood-sucking Rhodnius prolixus utilizes NO Protein Fe N NH CO₂ NO bound to NP1 (NP1-NO) NH NH₂ Histamine complexed with NO at pH 5.6. The haem unit is bound within a pocket in the protein comprising B-sheets and is tethered to the protein chain by a histidine residue. X-Ray crystallographic data are available on NP1-NO and its [CNJ analogue and confirm that NO and [CNJ bind to the haem Fe. However, in the X-ray beam, photoreduction of NPI-NO readily occurs, and it is difficult to assess whether or not the observed bent coordination mode of the NO ligand is a consequence of photo- reduction. The Fe-N-O bond angles differ between different structural determinations. For the cyanido complex, the Fe-C-N bond angle is 173°. The NO or [CN] ligand is 'lodged' in a pocket of the protein chain between two leucine residues (see Table 29.2). Structural data for the histamine complex show that this same protein pocket hosts the histamine ligand, indicating that NO and histamine compete for the same binding site. At physiological pH, the haem unit in NP1 binds histamine 100 times more strongly than NO. This should both aid the dissociation of NO and inhibit the role of histamine, both of which work in favour of the attacking Rhodnius prolixus.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)
a Both haemoglobin and cytochromes contain haemiron The presence of haemiron in both haemoglobin and cytochromes is related to their functions in biological processes involving oxygen and electron tra...View the full answer

Answered By

Diana Muriuki
As an online math tutor, I have several years of hands-on experience working with students of all ages and skill levels. I hold a Bachelor's degree in Mathematics and a Master's degree in Education. Additionally, I have completed multiple training courses in online teaching and tutoring methods.
Throughout my career, I have worked with students in both individual and group settings, including classroom teaching, after-school tutoring, and online instruction. I am proficient in teaching a wide range of math topics, from basic arithmetic to advanced calculus and statistics.
One of my greatest strengths as a tutor is my ability to adapt my teaching style to meet the unique needs and learning styles of each individual student. I understand that every student is different, and I strive to create a comfortable and supportive learning environment that encourages growth and development.
In addition to my formal education and tutoring experience, I am also a lifelong learner with a passion for mathematics. I am constantly seeking out new resources and methods to improve my own knowledge and skills, and I believe this passion and enthusiasm helps to inspire my students as well.
Overall, my hands-on experience and proficiency as a math tutor are grounded in a combination of formal education, practical experience, and a genuine love of mathematics. I am confident in my ability to help students achieve their goals and succeed in math, and I look forward to the opportunity to work with new students and continue to grow as an educator.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The Equity Premium Puzzle: Investments in equities (like stocks) yield substantially higher returns than investment in bonds. By itself, this is no surprisebecause stocks are riskier than bonds. What...
-
A school district superintendent wants to test a new method of teaching arithmetic in the fourth grade at his 15 schools. He plans to select 8 students from each school to take part in the...
-
1. Farmer Annie's Ice Cream Shop has calculated the marginal product of labor, and the results are shown in the following table. The wage for workers is $70 per day, and the pints of ice cream sell...
-
Big Burgers is in the fast-food restaurant business. One component of its marketing strategy is to increase sales by expanding in foreign markets. It uses both financial and nonfinancial quantitative...
-
In Exercises 1924, use the Leading Coefficient Test to determine the end behavior of the graph of the polynomial function. f(x) = 5x 4 + 7x 2 - x + 9
-
Show the impact of preexisting goodwill on the consolidation process. AppendixLO1
-
Northwest Aircraft Industries (NAI) was founded 45 years ago by Jay Preston as a small machine shop producing machined parts for the aircraft industry, which is prominent in the Seattle/Tacoma area...
-
The actual selling expenses incurred in March 2020 by Fallon Company are as follows. Variable Expenses Fixed Expenses Sales commissions $14,576 Sales salaries $34,700 Advertising 12,174 Depreciation...
-
(a) The compounds shown below are models for the active site of [FeFe]-hydrogenase. How are the models related to the active site and what problems does a crystallographer face when trying to...
-
(a) Whereas the stability constant, K, for the equilibrium: is of the order of 10, that for the equilibrium: is of the order of 3000. Rationalize this observation. (b) Photosystem II operates in...
-
A refrigerator runs on the vapor compression cycle, using HFC-134a as a refrigerant. The boiler operates at 20F. The effluent from the condenser is 10F above ambient temperature. The compressor has...
-
Evaluate the following integrals: (i) (ii) dx (x+1)(x+2) dx x(x+1)
-
Evaluate the following integrals:
-
Evaluate the following integrals:
-
Example: Evaluate each of the following: In(1+m) (a) e cos(1-e)dx 10- (b) [Int] -dt sec(3P)tan (3P) (c) 12 2+ sec(3P) dP (d) cos(x)cos(sin(x))dx (e) dw W 50 20
-
For the first 50 years of business the Johnson Carpet Company produced carpets for residential use. The salesforce was structured geographically. In the past 5 years a large percentage of carpet...
-
At 31 December 20X9, the end of the annual reporting period, the accounts of Huron Company showed the following: a. Sales revenue for 20X9, $ 2,950,000, of which one- quarter was on credit. b....
-
Predict the products for each of the following reactions: (a) (b) H2 Lindlar's catalyst :? H2 Pt -? Na NH, (1) ? Ni,B Pd Na NH3 (/)
-
Predict the products for each of the following reactions: H2 Lindlar's catalyst 2 Pt
-
Draw the products of each of the following acid-base reactions, and then predict the position of equilibrium in each case: (a) (b) NaH
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


