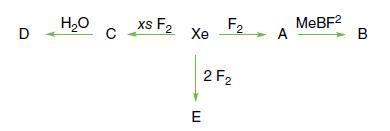
Identify the xenon compounds A, B, C, D, and E. D HO C xs F Xe F2
Question:
Identify the xenon compounds A, B, C, D, and E.
Transcribed Image Text:
D H₂O C xs F₂ Xe F2 2F₂ E A MeBF² B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Based on the provided information here are the identified ...View the full answer

Answered By

Akash M Rathod
I have been utilized by educators and students alike to provide individualized assistance with everything from grammar and vocabulary to complex problem-solving in various academic subjects. I can provide explanations, examples, and practice exercises tailored to each student's individual needs, helping them to grasp difficult concepts and improve their skills.
My tutoring sessions are interactive and engaging, utilizing a variety of tools and resources to keep learners motivated and focused. Whether a student needs help with homework, test preparation, or simply wants to improve their skills in a particular subject area, I am equipped to provide the support and guidance they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Understand what a stakeholder is and why stakeholder analysis might be important in the study of international business.
-
Calculate the Return on Invested Capital Cash Receivables Inventory Total current assets Net fixed assets Total assets Sales Cost of goods sold: Materials Labor Heat, light, and power Indirect labor...
-
if we choose statistic as our keyword, our cipher would be determined as follows: method i. write the word statistic without the repeated letters. then complete the cipher with the unused alphabet...
-
There are different types of non-probability sampling methods. Below are examples of such methods. You are required to go through them and (for each example); a. Name the Sample Method b. Write a not...
-
Troy Batkin, the chief executive officer of Batkin Corporation, has assembled his top advisers to evaluate an investment opportunity. The advisers expect the company to pay $400,000 cash at the...
-
The temperature of 3.0 kg of krypton gas is raised from 20 C to 80 C. (a) If this is done at constant volume, compute the heat added, the work done, and the change in internal energy. (b) Repeat if...
-
5. Let r = 0.08, S = $100, = 0, and = 0.30. Using the risk-neutral distribution, simulate 1/S1. What is E(1/S1)? What is the forward price for a contract paying 1/S1?
-
Xerox's iGenX high-speed commercial printers cost $1.5 billion to develop. The machines cost $500,000 to $750,000 depending on what options the client selects. Spectrum Imaging Systems is considering...
-
Given the following information for the Italia open pit gold mine, prepare annual cash flows for the expected life of the project. (10 Points) Grade of ore: 0.164 (troy) ounce/ ton Recovery: 92% Ore...
-
Draw the Lewis structures of (a) XeOF 4 , (b) XeO 2 F 2 , (c) XeO 6 2 .
-
In the paper Predicted chemical bonds between rare gases and Au (J. Am. Chem. Soc., 1995, 117, 2067), P. Pyykkii used a computational study of the species RgAuRg + and AuRg + (where Rg refers to a...
-
Steadman Company is considering an investment in a new machine for an independent five-year project. The machines cost is $500,000 with no salvage value at the end of five years. Net cash inflows...
-
Provide a brief bio of the leader and a brief overview of the change or crisis they led the organization or movement through. Discuss their leadership style during this change/crisis using one of the...
-
Write a C++ function named Ifsr that accepts feedback path and initial states as unsigned integers and the number of random bits to be printed as arguments. The function will print the random bits by...
-
Newfoundland Hapset will be remitted to _____
-
The Z Energy Corp. has a new investment opportunity that generates cash flows of $6 million per year (in expectation) forever. The managers of Z Energy are not sure what the required rate of return...
-
Define an interface TwoStrings Oper declaring a function apply which takes two strings and returns a string. Then, define four classes implementing this interface, where the operation on strings...
-
Explain the technical skill, human skill, and conceptual skill in an article.
-
Why do bars offer free peanuts?
-
(a) Which of the following compounds behave as acids in liquid HF: ClF 3 , BF 3 , SbF 5 , SiF 4 ? Write equations to explain this behaviour. (b) The salt [S 8 ][AsF 6 ] 2 can be isolated from the...
-
Early in the study of chemical reactions in liquid NH 3 , it was noted that nitrogen compounds behave in liquid NH 3 in a manner similar to analogous oxygen containing species in water. For example,...
-
Suggest explanations for the following observations. (a) In aqueous solution, AgNO 3 and KCl react to give a precipitate of AgCl, whereas in liquid NH 3 , KNO 3 and AgCl react to produce a...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...
-
Which of the following statements is not true regarding the $500 credit for dependent other than a qualifying child credit. Cannot be claimed on the same tax return if the child tax credit is also...
-
Grind Co. is considering replacing an existing machine. The new machine is expected to reduce labor costs by $127,000 per year for 5 years. Depreciation on the new machine is $57,000 compared with...

Study smarter with the SolutionInn App


