In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. Is it correct
Question:
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed.
Is it correct to interpret the phrase ‘static solution structure’ as meaning necessarily rigid? Use the following molecules to exemplify your answer: PMe3; OPMe3; PPh3; SiMe4.
Table 4.3
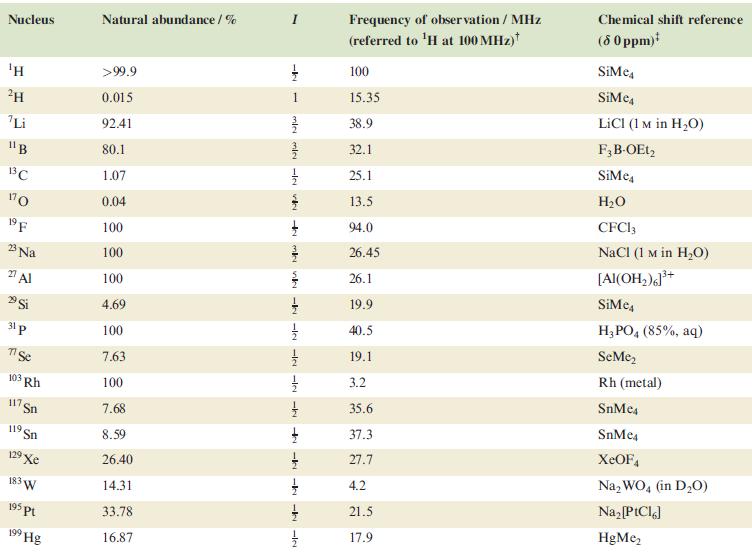
Transcribed Image Text:
Nucleus ΤΗ ²H Li 11B 13C 170 19 F 23 Na 2 Al 29 Si 31 p 7 Se 103 Rh 117. Sn 119 Sn 19 Xe 183 W 195 pt 199 Hg Natural abundance/% >99.9 0.015 92.41 80.1 1.07 0.04 100 100 100 4.69 100 7.63 100 7.68 8.59 26.40 14.31 33.78 16.87 miele v + mk nh -k -le -le-le - -ki -k -k -IN Frequency of observation / MHz (referred to ¹H at 100 MHz) 100 15.35 38.9 32.1 25.1 13.5 94.0 26.45 26.1 19.9 40.5 19.1 3.2 35.6 37.3 27.7 4.2 21.5 17.9 Chemical shift reference (8 0 ppm)* SiMe SiMe4 LICI (1 M in H₂O) F3B-OEt2 SiMe H₂O CFC13 NaCl (1 M in H₂O) [Al(OH₂)]³+ SiMc4 H3PO4 (85%, aq) SeMe₂ Rh (metal) SnMc4 SnMe4 XeOF4 Na₂WO4 (in D₂O) Na₂ [PtCl6] HgMe₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
The phrase static solution structure does not necessarily mean rigid It refers to the average structure of a molecule in solution as determined by met...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. The 19 F NMR spectrum of each of the following molecules exhibits one signal. For which species is this observation...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. (a) Predict the structure of SF 4 using the VSEPR model. (b) Account for the fact that at 298K and in solution the...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. Outline the mechanism of Berry pseudo-rotation, giving two examples of molecules that undergo this process. Table...
-
Mary Sue owns 600 shares of QRS Moving Company. QRS pays a quarterly dividend of $0.50 per share. What is the total annual dividend that Mary Sue will receive?
-
Rex Banner is the manager of a Stop Mart convenience store. He has been employed by the company for 12 years, the last 9 years as a store manager. Rex applied for a promotion to regional manager,...
-
Paint is poured onto a flat surface and a circular patch is formed. The area of the patch increases at a rate of 5cm 2 s 1 . a. Find, in terms of , the radius of the patch after 8 seconds. b. Find,...
-
Variable overheads vary with time. State whether the following statements are true or false:
-
Roberta Santos, age 41, is single and lives at 120 Sanbome Avenue, Springfield, IL 60781. Her Social Security number is 123-45-6789. Roberta has been divorced from her former husband, Wayne, for...
-
Siegmeyer Corp. is considering a new inventory system, Project A That will cost $800,000. The system is expected to generate positive cash flows over the next four years in the amounts of $350,000 in...
-
Use the VSEPR model to predict the structures of (a) H 2 Se, (b) [BH 4 ] , (c) NF 3 , (d) SbF 5 , (e) [H3O] + , (f) IF 7 , (g) [I 3 ] , (h) [I 3 ] + , (i) SO 3 .
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. The structure of [P5Br 2 ] + is shown in diagram 4.17. Account for the fact that the 31 P NMR spectrum of this...
-
If your preferred option involves a layoff, justify why. If it doesnt involve a layoff, explain why.
-
C 2 H 6 O 2 + NaOH + 6 H 2 O C 2 H 3 NaO 3 + O 2 + 3 H 2Hydrogen is produced at the cathode, oxYGEN AT THE ANODE .Mass balance to produce 5000 tonnes a year of glycolic acid, formic acid and oxalic...
-
Please answer: a discussion of the ethical issues involved. The court might not itself consider the ethics of the actions of the parties. However, I ask that you consider the ethics of the following:...
-
In Exercises 21-24, use these results from the "1-Panel-THC" test for marijuana use, which is provided by the company Drug Test Success: Among 143 subjects with positive test results, there are 24...
-
I need help for an assignment of a review on research on Virtual Education on study motivation and academic performance in university students. I am attaching a research article from a magazine to...
-
Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this small...
-
What requirements must be satisfied to analyze a randomized complete block design?
-
Refer to the data in QS 10-1. Based on financial considerations alone, should Helix accept this order at the special price? Explain.
-
Sketch the two possible geometric isomers of the octahedral [AsF 4 Cl 2 ] and explain how they could be distinguished by 19 F-NMR.
-
The tetrahedral P 4 molecule may be described in terms of localized 2c,2e bonds. Determine the number of skeletal valence electrons and from this decide whether P 4 is closo, nido, or arachno. If it...
-
Calculate the standard potential of the reaction of H 3 PO 2 with Cu 2+ . Are HPO 2 2 and H 2 PO 2 2 useful as oxidizing or as reducing agents?
-
In the context of portfolio theory, what is diversification primarily intended to do ? A ) Increase returns. B ) Reduce risk. C ) Maximize tax efficiency. D ) Simplify investment management.
-
4. The risk-free rate of return is 3.78% and the market risk premium is 6.42%. What is the expected rate of return on a stock with a beta of 1.09?
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...

Study smarter with the SolutionInn App


