In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. (a) Predict the
Question:
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed.
(a) Predict the structure of SF4 using the VSEPR model.
(b) Account for the fact that at 298K and in solution the 19F NMR spectrum of SF4 exhibits a singlet but that at 175 K, two equal-intensity triplets are observed.
Table 4.3
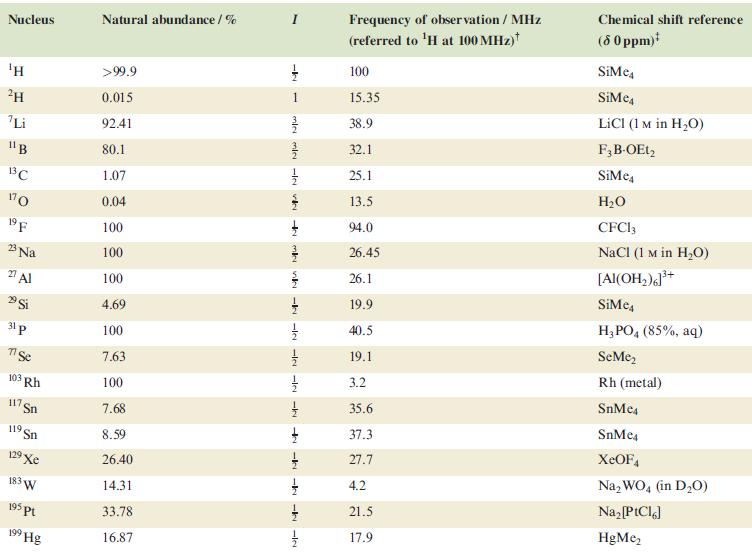
Transcribed Image Text:
Nucleus ΤΗ ²H Li 11B 13C 170 19 F 23 Na 2 Al 29 Si 31 p 7 Se 103 Rh 117. Sn 119 Sn 19 Xe 183 W 195 pt 199 Hg Natural abundance/% >99.9 0.015 92.41 80.1 1.07 0.04 100 100 100 4.69 100 7.63 100 7.68 8.59 26.40 14.31 33.78 16.87 miele v + mk nh -k -le -le-le - -ki -k -k -IN Frequency of observation / MHz (referred to ¹H at 100 MHz) 100 15.35 38.9 32.1 25.1 13.5 94.0 26.45 26.1 19.9 40.5 19.1 3.2 35.6 37.3 27.7 4.2 21.5 17.9 Chemical shift reference (8 0 ppm)* SiMe SiMe4 LICI (1 M in H₂O) F3B-OEt2 SiMe H₂O CFC13 NaCl (1 M in H₂O) [Al(OH₂)]³+ SiMc4 H3PO4 (85%, aq) SeMe₂ Rh (metal) SnMc4 SnMe4 XeOF4 Na₂WO4 (in D₂O) Na₂ [PtCl6] HgMe₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
a To predict the structure of SF using the VSEPR model Valence Shell Electron Pair Repulsion model we first determine the valence electron count of the sulfur S and fluorine F atoms Sulfur is in group ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
VSEPR (valence state electron pair repulsion) theory was formulated to anticipate the local geometry about an atom in a molecule (see discussion in Section 25.1). All that is required is the number...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. NaBH 4 contains the tetrahedral [BH4] ion. Although NaBH 4 hydrolyses slowly in water, it is possible to obtain a...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. Rationalize the fact that the 13 C NMR spectrum of CF 3 CO 2 H consists of two binomial quartets with coupling...
-
Two forces are acting on the 5 kg object, with only one of them shown in Fig. 1. Knowing that F = 20 N, 0 = 30 and that the object is moving at a constant acceleration of a = 12 m/s, find the second...
-
Jake needed a summer job and was lucky enough to land a position as a ticket collector at a local amusement park. On his first day, he was assigned to work alongside Tim who had worked at the park...
-
The inside lane of a school running track consists of two straight sections each of length x metres, and two semicircular sections each of radius r metres, as shown in the diagram. The straight...
-
Directors remuneration and expenses form a part of administrative overheads. State whether the following statements are true or false:
-
Two very narrow slits are spaced 1.80m apart and are placed 35.0 cm from a screen. What is the distance between the first and second dark lines of the interference pattern when the slits are...
-
Consider the following scenario: You are an assistant to the accounting manager for a small company that sells sports equipment online. The manager needs analysis of the sales by-product to be...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. The structure of [P5Br 2 ] + is shown in diagram 4.17. Account for the fact that the 31 P NMR spectrum of this...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. Outline the mechanism of Berry pseudo-rotation, giving two examples of molecules that undergo this process. Table...
-
Matt Henry owns a business called Henry's Sporting Goods. His beginning inventory as of January 1, 20--, was $45,000, and his ending inventory as of December 31, 20--, was $57,000. Set up T-accounts...
-
Briefly, discuss the use of survey research in exploratory, descriptive, explanatory, and evaluation studies. Using a criminal justice example select one type of research study and develop one...
-
Medical Helicopters In a study of helicopter usage and patient survival, results were obtained from 47,637 patients transported by helicopter and 111,874 patients transported by ground (based on data...
-
Woodland Hills Company reported income before taxes (pretax financial income) in its income statement of $60,000. Among the items included in the computation of pretax financial income were the...
-
cest Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this...
-
The activation energy for the gas phase decomposition of isobutyl bromide is 211 kJ. (CH3)2CHCH2 Br (CH3)2C=CH2+ HBr The rate constant at 676 K is 5.73 x 10-4 s. The rate constant will be 0.00647 s...
-
David Elkington, the Chief Executive Officer (CEO) of InsideSales.com, wondered whether the cycles of the moon affect sales. He got the idea from his father-in-law, who said that emergency rooms are...
-
An access route is being constructed across a field (Figure Q8). Apart from a relatively firm strip of ground alongside the field's longer side AB, the ground is generally marshy. The route can...
-
When equal volumes of nitric oxide (NO) and air are mixed at atmospheric pressure a rapid reaction occurs, to form NO 2 and N 2 O 4 . However, nitric oxide from an automobile exhaust, which is...
-
Are reactions of NO 2 as an oxidizing agent generally faster or slower when pH is lowered? Give a mechanistic explanation for the pH dependence of NO 2 oxidations.
-
Write the balanced chemical equation corresponding to the standard enthalpy of formation of P 4 O 10 (s). Specify the structure, physical state (s, l, or g), and allotrope of the reactants. Does...
-
help me A 35% discount on 3 smart phone amounts to $385. What is the phone's list price? Answer =$ (rounded to the nearest cent)
-
What effect is there on the income statement and balance sheet when an expense is left too long as a liability
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid

Study smarter with the SolutionInn App


