In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. Rationalize the fact
Question:
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed.
Rationalize the fact that the 13C NMR spectrum of CF3CO2H consists of two binomial quartets with coupling constants of 44 and 284 Hz respectively.
Table 4.3
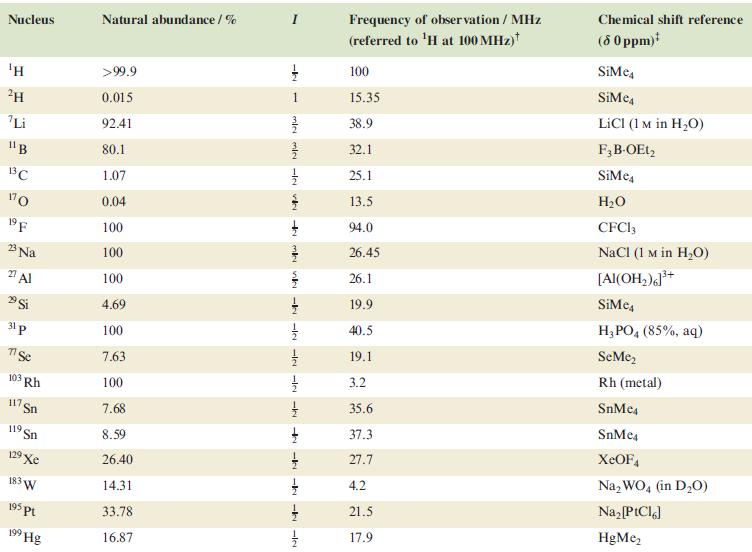
Transcribed Image Text:
Nucleus ΤΗ ²H Li 11B 13C 170 19 F 23 Na 2 Al 29 Si 31 p 7 Se 103 Rh 117. Sn 119 Sn 19 Xe 183 W 195 pt 199 Hg Natural abundance/% >99.9 0.015 92.41 80.1 1.07 0.04 100 100 100 4.69 100 7.63 100 7.68 8.59 26.40 14.31 33.78 16.87 miele v + mk nh -k -le -le-le - -ki -k -k -IN Frequency of observation / MHz (referred to ¹H at 100 MHz) 100 15.35 38.9 32.1 25.1 13.5 94.0 26.45 26.1 19.9 40.5 19.1 3.2 35.6 37.3 27.7 4.2 21.5 17.9 Chemical shift reference (8 0 ppm)* SiMe SiMe4 LICI (1 M in H₂O) F3B-OEt2 SiMe H₂O CFC13 NaCl (1 M in H₂O) [Al(OH₂)]³+ SiMc4 H3PO4 (85%, aq) SeMe₂ Rh (metal) SnMc4 SnMe4 XeOF4 Na₂WO4 (in D₂O) Na₂ [PtCl6] HgMe₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
To rationalize the fact that the 13C NMR spectrum of CF3CO2H trifluoroacetic acid consists of two binomial quartets with coupling constants of 44 and ...View the full answer

Answered By

User l_917591
As a Business Management graduate from Moi University, I had the opportunity to work as a tutor for undergraduate students in the same field. This experience allowed me to apply the theoretical knowledge I had gained in a practical setting, while also honing my teaching and communication skills.
As a tutor, I was responsible for conducting tutorial sessions, grading assignments and exams, and providing feedback and support to my students. I also assisted with the preparation of course materials and collaborated with other tutors and professors to ensure consistency in teaching and assessment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. The 19 F NMR spectrum of the octahedral ion [PF5Me] shows two signals ( 45.8 and 57.6 ppm). Why are two signals...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. (a) Predict what you would expect to see in the 15 N NMR spectrum of the isotopically labelled compound cis-[Pt( 15...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. NaBH 4 contains the tetrahedral [BH4] ion. Although NaBH 4 hydrolyses slowly in water, it is possible to obtain a...
-
What is the time value of money? Including present and future value. How would a financial expert interpret it?
-
What does the term free on board mean? Why is this an important term to understand if you are involved in making decisions about the purchasing of inventory or the setting of prices for your products?
-
Suppose you have an oracle, OM(s), that correctly predicts the opponents move in any state. Using this, formulate the definition of a game as a (single-agent) search problem. Describe an algorithm...
-
The oak was purchased in 16-foot lengths and inspected for any flaws or excessive knots to ensure it was #1 grade. LO.1
-
A company is considering two mutually exclusive expansion plans. Plan A requires a $40 million expenditure on a large-scale integrated plant that would provide expected cash flows of $6.4 million per...
-
According to the global minimum wage graph, name two countries with higher minimum wages than the US. Name two countries with lower minimum wages than the US.
-
In the MALDI-TOF mass spectrum of the macrocyclic ligand shown below in 1,8,9-trihydroxyanthracene matrix, the dominant peaks are at m/z 615.7 (base peak) and 637.7. Assign the peaks HN NH HN - NH N...
-
The UV-VIS spectrum of a CH 2 Cl 2 solution of the gold (I) compound shown below with R = Ph is: max () = 239 (92500), 269 (67 000), 286 (72 000), 303 (28 000), 315 nm (21000 dm 3 mol 1 cm 1 ). (a)...
-
Presented below is information related to Williams and Douglas, Attorneys at Law. Retained earnings, January 1, 2014 ......$ 23,000 Legal service revenue2014 ......... 340,000 Total expenses2014...
-
Sketch plane / intersecting plane K. Then draw a line & in plane J that intersects plane Kat a single point. A X C B D E
-
Use a graphing utility to verify any five of the graphs that you drew by hand in Exercises 126. Data from exercise 1-26 1. x + 2y = 8 3. x2y> 10 2. 3x6y 12 4. 2xy > 4
-
The following information pertains to Porter Company for 2011. Ending inventory consisted of 30 units. Porter sold 320 units at \(\$ 30\) each. All purchases and sales were made with cash. Required...
-
In Problems 7780, use a numerical integration routine on a graphing calculator to find the area bounded by the graphs of the indicated equations over the given interval (when stated). Compute answers...
-
Solar Heating, Inc., had the following transactions for 2011: Required a. Determine the quantity and dollar amount of inventory at the end of the year, assuming Solar Heating Inc. uses the FIFO cost...
-
The EAS Temperament Survey can be used to measure the activity, emotionality, and sociability of adults. In a study on the effects of emotions on time perception, a researcher asked subjects to...
-
Given find the value of k. es 1 e kx dx = 1 4'
-
Determine whether the number of IR and Raman active stretching modes could be used to determine uniquely whether a sample of gas is BF 3 , NF 3 , or ClF 3 .
-
Determine the symmetry elements and assign the point group of (a) NH 2 Cl, (b) CO 3 2 , (c) SiF 4 , (d) HCN, (e) SiFClBrI, (f) BF 4 .
-
Group theory is often used by chemists as an aid in the interpretation of IR spectra. For example, there are four NH bonds in NH 4 + and four stretching modes are possible. There is the possibility...
-
En prenant un exemple de votre choix, montrer comment on value un swap de taux de change.
-
How much would you need to invest today in order to receive: a. $10,000 in 5 years at 11%? b. $11,000 in 12 years at 8%? c. $12,000 each year for 10 years at 8%? d. $12,000 at the beginning of each...
-
A company that manufactures pulse Doppler insertion flow meters uses the Straight Line method for book depreciation purposes. Newly acquired equipment has a first cost of $190,000 with a 3-year life...

Study smarter with the SolutionInn App


