Many of the tabulated data for standard potentials have been determined from thermochemical data rather than direct
Question:
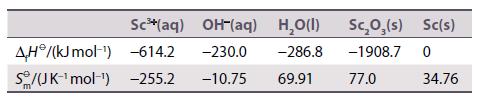
Many of the tabulated data for standard potentials have been determined from thermochemical data rather than direct electrochemical measurements of cell potentials. Carry out a calculation to illustrate this approach for the half-reaction
![]()
Transcribed Image Text:
Sc₂O₂ (s) + 3H₂O(l) +6e2Sc(s) + 6OH-(aq).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
To illustrate the approach of determining standard potentials from thermochemical data we can u...View the full answer

Answered By

Akash M Rathod
I have been utilized by educators and students alike to provide individualized assistance with everything from grammar and vocabulary to complex problem-solving in various academic subjects. I can provide explanations, examples, and practice exercises tailored to each student's individual needs, helping them to grasp difficult concepts and improve their skills.
My tutoring sessions are interactive and engaging, utilizing a variety of tools and resources to keep learners motivated and focused. Whether a student needs help with homework, test preparation, or simply wants to improve their skills in a particular subject area, I am equipped to provide the support and guidance they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
X-rays of wavelength 0.0973 nm are directed at an unknown crystal. The second diffraction maximum is recorded when the X-rays are directed at an angle of 23.4 relative to the crystal surface. What is...
-
Why might Mattel set a much lower contribution margin on its Barbie dolls than on the accessories for the dolls?
-
Repeat the calculations of Prob. 1552 for several angles of attack of the heating elements, from 0 (horizontal) to 90 (vertical). Use identical inlet conditions and wall conditions for each case....
-
More on mens heights. The distribution of heights of young men is approximately Normal with mean 70 inches and standard deviation 2.5 inches. Use the 689599.7 rule to answer the following questions....
-
Robert in Chicago entered into a contract to sell certain machines to Terry in New York. The machines were to be manufactured by Robert and shipped F.O.B. Chicago not later than March 25. On March...
-
Michael Ltd. reported following transactions and information regarding the common shares of Mitchell Company: March 1, 2020: Purchased 50,000 shares at $26.75 plus $12,000 commission. December 31,...
-
Data shown below refer to redox couples for the Group 8 elements Fe and Ru. (a) Comment on the relative stability of Fe 2+ and Ru 2+ in acidic aqueous solution. (b) Give a balanced equation for the...
-
Reduction potentials for half-cell reactions of simple carbon species measured in aqueous solution at pH 7.0, 25C, are as follows. (a) Assign carbon oxidation numbers to each of the species involved....
-
Can a diode be made from material that is doped with just one type of impurity atom? Explain.
-
Among 450 randomly selected drivers in the 16 - 18 age bracket, 374 were in a car crash in the last year. If a driver in that age bracket is randomly selected, what is the approximate probability...
-
Construct a 90% confidence interval for the population standard deviation o at Bank A. Bank A 6.4 6.6 6.7 6.8 7.1 7.2 7.6 7.8 7.8 7.8
-
In 2002, after the accounting deceptions of the management of many multi-million dollar corporations (with Enron being the benchmark name of that time period), the Security and Exchange Commission...
-
1.Deduce the structure of a compound with molecular formula CsH100 that exhibits the following IR, H NMR, and 13C NMR spectra. Data from the mass spectrum are also provided. Mass Spec. Data relative...
-
Transcribed image text: Prots Caco.ch Part 2 Income Statement Med Earningstemet Tante Sheet For the event.com Competence ended The fram C an an dy wana A TO nede ANG ore.com wwwwww og for to...
-
In cryptography, cipher text is encrypted or encoded text that is unreadable by a human or computer without the proper algorithm to decrypt it into plain text. The impact of erroneous cipher texts on...
-
As indicated by mutual fund flows, investors tend to beat the market seek safety invest in last year's winner invest in last years loser
-
The first step in the Cativa process is the reaction between MeI and cis-[Ir(CO) 2 I 2 ] . However, the catalyst may also react with HI and this step initiates a water gas shift reaction that...
-
Describe briefly why a clean nickel surface (fcc structure) should not be regarded as comprising a perfect close-packed array of atoms. Indicate the arrangements of atoms that an adsorbate might...
-
Give a brief discussion of the use of homogeneous catalysis in selected industrial manufacturing processes.
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


