One member of each of the following sets of compounds is not isoelectronic with the others. Which
Question:
One member of each of the following sets of compounds is not isoelectronic with the others. Which one in each set is the odd one out?
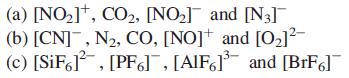
Transcribed Image Text:
(a) [NO₂], CO₂, [NO₂] and [N3] (b) [CN], N₂, CO, [NO] and [0₂]²- (c) [SiF6], [PF6], [AlF6]³ and [BrF]¯¯
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
To identify the odd one out in each set of compounds based on isoelectronic species we need to compa...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Googles ease of use and superior search results have propelled the search engine to its num- ber one status, ousting the early dominance of competitors such as WebCrawler and Infos- eek. Even later...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
2. Evaluate the following definite integrals (a) (2x - 6x) dx /6 (b) 16 cos 3t + 2 sin 3t dt x+1 2 da
-
Liberty Advertising, Inc., engaged in the following business transactions during July of 2012: Jul 1 Borrowed $325,000 from Oakville Bank. The company president signed a note payable to the bank in...
-
Suppose the range of the nuclear force was 5 fm. Compute the mass (in MeV/c 2 ) of an exchange particle that might mediate such a force.
-
1. How are enterprise and internal service funds similar? How are they different?
-
Fashion Standards is a retail clothing store. Sales of merchandise and purchases of goods on account for January 2016, the first month of operations, appear below. INSTRUCTIONS 1. Record the...
-
At time t the one-year interest rate is 5%, the two-year rate is 5.75%, and the liquidity premium is 0.25%. Using the liquidity premium theory, solve for the forward rate one year from now.
-
Using the data in Table 2.2, determine which of the following covalent single bonds is polar and (if appropriate) in which direction the dipole moment acts. (a) NH; (b) FBr; (c) CH; (d) PCl; (e) NBr....
-
Draw Lewis structures to describe the bonding in the following molecules: (a) F 2 ; (b) BF 3 ; (c) NH 3 ; (d) H 2 Se; (e) H 2 O 2 ; (f) BeCl 2 ; (g) SiH 4 ; (h) PF 5 .
-
Article 2 of the UCC governs contracts, while Article 2A governs contracts. a. Lease; sales b. Sales; lease c. Service; sales d. Sales; service e. Service; leasePage 238
-
Presented below is information related to Rembrandt Inc's inventory, assuming Rembrandt uses lower-of-LIFO cost- or-market. (per unit) Skis Boots Parkas Historical cost $190.00 $106.00 $53.00 Selling...
-
7. Below is a UML model describing a typical organization of class modules taken by students: A student can take several modules. (Note that a module is offered even if it is not taken by any...
-
Beswick Limited manufactures mountain and road bikes. The trial balance at 3 1 December 2 0 2 0 was as follows: Dr Cr Revenue 3 , 5 6 4 , 3 0 0 Purchases 1 , 5 7 8 , 2 5 0 Inventory on 3 1 / 1 2 / 1...
-
Kubin Company's relevant range of production is 10,000 to 12,000 units. When it produces and sells 11,000 units, its average costs per unit are as follows: Average Cost per Unit $ 7.10 Direct...
-
Smithen Company, a wholesale distributor, has been operating for only a few months. The company sells three products-sinks, mirrors, and vanities. Budgeted sales by product and in total for the...
-
In the European Union, it has become important to be able to determine an individual's age when legal documentation of the birth date of an individual is unavailable. In the article "Age Estimation...
-
The outer loop controls the number of students. Note that the inner loop of this program is always executed exactly three times, once for each day of the long weekend. Modify the code so that the...
-
In their article A thermochemical study of ceria: exploiting an old material for new modes of energy conversion and CO 2 mitigation (Philos. Trans. R. Soc. London, 2010, 368, 3269), Chueh and Haile...
-
Using the following aqueous acid solution reduction potentials E (Pd 2+ ,Pd) = +0.915 V and E ([PdCl 4 ] 2 ,Pd) = +0.60 V, calculate the equilibrium constant for the reaction Pd+ (aq) + 4 Cl(aq)...
-
Draw a Frost diagram for mercury in acid solution, given the following Latimer diagram: Comment on the tendency of any of the species to act as an oxidizing agent, a reducing agent, or to undergo...
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


