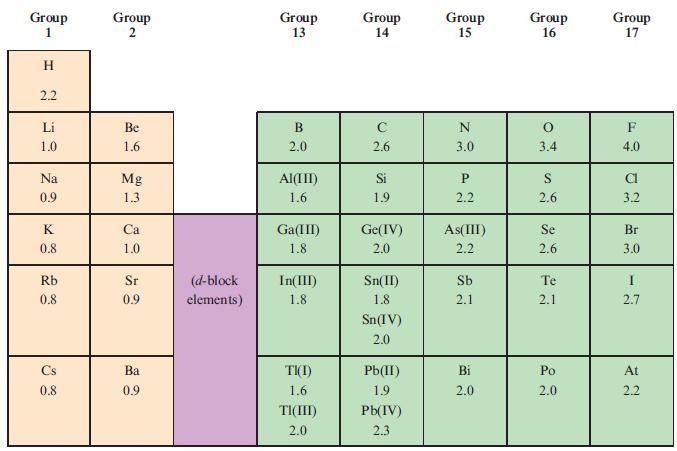
Using the data in Table 2.2, determine which of the following covalent single bonds is polar and
Question:
Using the data in Table 2.2, determine which of the following covalent single bonds is polar and (if appropriate) in which direction the dipole moment acts.
(a) N—H;
(b) F—Br;
(c) C—H;
(d) P—Cl;
(e) N—Br.
Table 2.2
Transcribed Image Text:
Group 1 I H 2.2 Li 1.0 Na 0.9 K 0.8 Rb 0.8 Cs 0.8 Group 2 Be 1.6 Mg 1.3 Ca 1.0 Sr 0.9 Ba 0.9 (d-block elements) Group 13 B 2.0 Al(III) 1.6 Ga(III) 1.8 In(III) 1.8 TI(I) 1.6 TI(III) 2.0 Group 14 с 2.6 Si 1.9 Ge(IV) 2.0 Sn(II) 1.8 Sn(IV) 2.0 Pb(II) 1.9 Pb(IV) 2.3 Group 15 N 3.0 P 2.2 As(III) 2.2 Sb 2.1 Bi 2.0 Group 16 O 3.4 S 2.6 Se 2.6 Te 2.1 Po 2.0 Group 17 F 4.0 CI 3.2 Br 3.0 I 2.7 At 2.2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a NH Electronegativity difference 304 220 084 Since the electronegativity di...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using the data in Table 116 on page 300, indicate the semiannual interest payment dates for the Motorola bonds that mature in 2031. (For the item in question, look under Interest Dates.) The two...
-
Using the data in Table 4-3, calculate the G° for ring flip to the other conformation of the molecules depicted in Problem 30. Make sure that the sign (i.e., positive or negative) of your values...
-
Using the data in the following table, predict the sign and magnitude of ÎH° for each of the following reactions. In each case, identify whether the reaction is expected to be endothermic...
-
The total cost of 2 chair and 1 table is 210 dollars. The total cost of 1 chair and 2 tables is 285 dollars. What is the cost of 1 chair?
-
Tucker Stevens opened an accounting firm on October 1, 2012. During the month of October, the business completed the following transactions: Oct 1 The business sold $50,000 of common stock to open...
-
The half-life of 227 Th is 18.72 days. It decays by a emission to 223 Ra, an a emitter whose half-life is 11.43 days. A particular sample contains 10 6 atoms of 227 Th and no 223 Ra at time t = 0....
-
3 When managers obtain an expatriate posting abroad they sometimes need to change their natural management style to be successful. Expand the argument, giving examples of situations where a new...
-
A survey of 1,085 adults asked, Do you enjoy shopping for clothing for yourself? The results (data extracted from Split Decision on Clothes Shopping, USA Today, January 28, 2011.) indicated that 51%...
-
july 1 st k . samuels, sole shareholder of samuels internet cafe ltd , started his business by investing by investing his cash saving of $ 8 2 0 0 for 1 0 0 shares of the business . 1 st , Recevied a...
-
Use the Lewis structure model to deduce the type of nitrogennitrogen bond present in (a) N 2 H 4 , (b) N 2 F 4 , (c) N 2 F 2 (d) [N 2 H 5 ] + .
-
One member of each of the following sets of compounds is not isoelectronic with the others. Which one in each set is the odd one out? (a) [NO], CO, [NO] and [N3] (b) [CN], N, CO, [NO] and [0]- (c)...
-
In Problems 3336, there is a tie for the choice of the first pivot column. Use the simplex method to solve each problem two different ways: first by choosing column 1 as the first pivot column, and...
-
San Antonio S.A. rents a store in the Cusco Shopping Center, carrying out a series of modifications and installations in said store with the commitment that, at the end of the rental, it will...
-
b. If the above transactions covered a full year's operations, prepare a journal entry to dispose of the overhead account balance. Assume that the balance is significant. Also assume that the...
-
On 1 May 2015 Harry's Plastics Ltd acquires goods from a supplier in the US. The goods are shipped f.o.b. from the United States on 1 May 2015. The cost of the goods is US$1 500 000. The amount has...
-
In this assignment, you are going to analyze the financial viability of two companies, currently listed on the TSX . Then you will make an investment decision and justify your reasoning. Email your...
-
Create a journal entry for expense closing enteries. Time period: 3 months Entry number HBS073 This journal entry have 13 accounts Income Statement Weeks 1-10 Total Revenue Rental Revenue Sales...
-
In the article "Bigger Teeth for Longer Life? Longevity and Molar Height in Two Roe Deer Populations" (Biology Letters [June, 2007] vol. 3 no. 3 268-270), researchers developed a model to predict the...
-
In muscle tissue, the ratio of phosphorylase a to phosphorylase b determines the rate of conversion of glycogen to glucose 1phosphate. Classify how each event affects the rate of glycogen breakdown...
-
Consult the Ellingham diagram in Fig. 6.16 and determine if there are any conditions under which aluminium might be expected to reduce MgO. Comment on these conditions. Figure 6.16. A,G*/ (kJ md-)...
-
In Fig. 6.11, which of the boundaries depend on the choice of Fe 2+ concentration as 10 5 mol dm 3 ? Figure 6.11. +0.8 +0.4 E/V -0.4 -0.8 Fe3+ Fe2+ H,O/H, 024 Fe(OH),(s) Fe(OH), (s) 6 8 10 12 14 pH
-
Adding NaOH to an aqueous solution containing Ni 2+ results in precipitation of Ni(OH) 2 . The standard potential for the Ni 2+ /Ni couple is 0.25 V and the solubility product K sp = [Ni 2+ ] [OH ]...
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


