Reaction of H 3 L (drawn below) with Cu(O 2 CMe) 2 H 2 O in
Question:
Reaction of H3L (drawn below) with Cu(O2CMe)2 · H2O in MeOH with addition of pyridine (py) yields [Cu4L2(O2CMe)2(py)4(MeOH)2]. Show that a MALDI-TOF mass spectrum with peak envelopes at m/z 977 and 611 is consistent with this formulation.
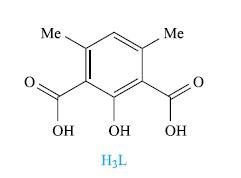
Transcribed Image Text:
Me ОН ОН H₂L Me ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
To determine if the MALDITOF mass spectrum with peak envelopes at mz 977 and 611 is consistent with ...View the full answer

Answered By

Mary Boke
I have teached the student upto class 12th as well as my fellow mates.I have a good command in engineering,maths and science.I scored 90+ marks in 10th and 12th in maths.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In 2002, workers at the Swedish National Food Administration discovered that heated, carbohydrate-rich foods, such as french fries, potato chips, and bread, contain alarming levels (0.1 to 4 g/g) of...
-
A graduate student was following a procedure to make 3-propylcyclohexa-1,4-diene. During the workup procedure, his research adviser called him into her office. By the time the student returned to his...
-
Hart and Grant formed Hart Limited Partnership. Hart put in a capital contribution of $20,000 and became a general partner. Grant put in a capital contribution of $10,000 and became a limited...
-
Pinduoduo plans to launch a new platform to compete with its competitors, JD.Com, Taobao, and Tmall. The product manager of the new platform should decide whether to leverage the social commerce...
-
Waters Landscaping, Inc., completed the following transactions during its first month of operations for January 2012: a. Gary Waters invested $7,500 cash and a truck valued at $1 5,000 to start...
-
The resurgence of interest in AI in the 2010s is often attributed to deep learning. Explain what deep learning is, how it relates to AI as a whole, and where the core technical ideas actually...
-
The approved boards were run through a multihead mill that planed the bottom side and shaped the top and sides of the handrail? LO.1
-
A gas containing methane, ethane, and carbon dioxide is analyzed with a gas chromatograph (GC) and flame ionization detector (FID): the GC separates the components of the gas and the FID registers...
-
Famas Llamas has a weighted average cost of capital of 11 percent. The companys cost of equity is 13 percent, and its pretax cost of debt is 9 percent. The tax rate is 40 percent. What is the...
-
The UV-VIS spectrum of a CH 2 Cl 2 solution of the gold (I) compound shown below with R = Ph is: max () = 239 (92500), 269 (67 000), 286 (72 000), 303 (28 000), 315 nm (21000 dm 3 mol 1 cm 1 ). (a)...
-
Open the structure file for problem 3.41: this shows the structure of -P 4 S 3 . (a) Orientate the structure so that the unique P atom is closest to you and the P 3 triangle coincides with the plane...
-
As stated in Chapter 23, mammalian cells can become resistant to the lethal action of methotrexate by the selective survival of cells containing increases in dihydrofolate reductase gene copy number...
-
Aircraft \(B\) has a constant speed of \(150 \mathrm{~m} / \mathrm{s}\) as it passes the bottom of a circular loop of 400-m radius. Aircraft \(A\) flying horizontally in the plane of the loop passes...
-
A small inspection car with a mass of \(200 \mathrm{~kg}\) runs along the fixed overhead cable and is controlled by the attached cable at \(A\). Determine the acceleration of the car when the control...
-
An aircraft \(P\) takes off at \(A\) with a velocity \(v_{0}\) of \(250 \mathrm{~km} / \mathrm{h}\) and climbs in the vertical \(y^{\prime}-z^{\prime}\) plane at the constant \(15^{\circ}\) angle...
-
If each resistor in Figure P31.75 has resistance \(R=5.0 \Omega\), what is the equivalent resistance of the combination? Data from Figure P31.75 wwwwww wwwww www www wwwww
-
Identify the proper point to recognize expense for each of the following transactions. a. Kat Inc. purchases on credit six custom sofas for \(\$ 800\) each in June. Two of the sofas are sold for \(\$...
-
Explain the rationale behind the decision rule for the Mann-Whitney test?
-
Could the owner of a business prepare a statement of financial position on 9 December or 23 June or today?
-
Consider a molecule IF 3 O 2 (with I as the central atom). How many isomers are possible? Assign point group designations to each isomer.
-
Do the hypothetical species (a) Square H 4 2+ , (b) Angular O 3 2 have a duplet or octet of electrons? Explain your answer and decide whether either of them is likely to exist.
-
Construct and label molecular orbital diagrams for N 2 , NO, and O 2 showing the principal linear combinations of atomic orbitals being used. Comment on the following bond lengths: N 2 110 pm, NO 115...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...

Study smarter with the SolutionInn App


