Substitution of H 2 O in [Fe(OH 2 ) 6 ] 3+ by thiocyanate is complicated by
Question:
Substitution of H2O in [Fe(OH2)6]3+ by thiocyanate is complicated by proton loss. By considering the reaction scheme below, derive an expression for
![]()
in terms of the equilibrium and rate constants, [Fe(OH2)63+], [SCN]−, [Fe(OH2)5(SCN)2+] and [H+].
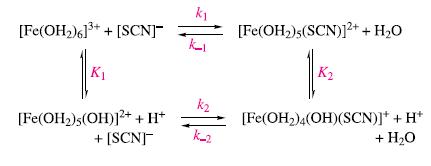
Transcribed Image Text:
d[SCN] dt
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To derive an expression for the rate of the reaction involving the substitution of H2O in FeOH263 by thiocyanate SCN in the presence of protons H we n...View the full answer

Answered By

Mary Boke
I have teached the student upto class 12th as well as my fellow mates.I have a good command in engineering,maths and science.I scored 90+ marks in 10th and 12th in maths.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The net potential energy EN between two adjacent ions is sometimes represented by the expression in which r is the interionic separation and C, D, and Ï are constants whose values depend on the...
-
You start driving east for 14 miles, turn left, and drive north for another 10 miles. At the end of driving, what is your straight line distance from your starting point? Round to the nearest tenth...
-
Flowers Associates is evaluating the performance of three divisions: Daisies, Pansies, and Tulips. Using the data that follow, compute the return on investment and residual income for each division,...
-
Each table of values gives several points that lie on a line.(a) What is the x-intercept of the line? The y-intercept?(b) Which equation in choices AD corresponds to the given table of values?(c)...
-
What is an organizational goal and in what ways is it important? LO.1
-
The adjusted trial balances of Verne Corporation at August 31, 2018, and August 31, 2017, include these amounts (in millions): Verne completed these transactions (in millions) during the year ended...
-
Workers in the U . S . pay several types of taxes on income. The lesson discussed the FICA taxes. You also have to pay federal income tax. Your federal income tax rate is based on the amount of money...
-
Rationalize the observation that when the reaction: is carried out in H 2 ( 18 O), the water in the complex contains equal proportions of H 2 ( 18 O) and H 2 ( 16 O). [Co(NH3)4 (CO3)]* [HO]*, HO 13+...
-
(a) Rationalize the formation of the products in the following sequence of reactions: (b) Suggest methods of preparing [RhCl 5 (OH 2 )] 2 , cis-[RhCl 4 (OH 2 ) 2 ] and fac-[RhCl 3 (OH 2 ) 3 ]....
-
Problem refer to the following Venn diagram. Which of the numbers x, y, z, or w must equal 0 if A B = A B? A B y
-
Link two articles from a trade journal in your field and discuss them in a short paper...
-
Evaluate vendors for supplying differentiated or specialized widgets. Review the "Supplier Scorecard Data" document and answer the questions below. 1. Discuss which supplier you will select,...
-
3. By keeping the leading term in the relativistic correction, the kinetic energy operator T of a relativistic electron in one dimension can be written as p 3p4 + 2m 8m3c2 where c is the speed of...
-
CHOOSE CORRECT OPTION How might inadequate training impact Thandiwe's ability to address classroom challenges? a. Develops effective teaching strategies b. Enhances problem solving skills and creates...
-
A 3.5-kg cannon on wheels is loaded with a 0.0527-kg ball. The cannon and ball are initially moving forward with a speed of 1.27 m/s. The cannon is ignited and launches a 0.0527-kg ball forward with...
-
Mountain Extreme manufactures mountain biking clothes and shoes. The company has two product lines (clothing and shoes), which are produced in separate manufacturing facilities; however, both...
-
Willingness to pay as a measure of a person's value for a particular good measures the maximum a person would be willing to pay requires that payment actually be made depends on the satisfaction that...
-
Draw a plausible mechanism for the following transformation: , [H2SO4]
-
Compound A has molecular formula C 8 H 14 O 2 . Upon treatment with catalytic acid, compound. A is converted into the cyclic hemiacetal. Identify the structure of compound A. , Compound A [H*]
-
Draw a plausible mechanism for each of the following transformations: (a) (b) [TSOH] MENH2 -H20 Et [TSOH] EENH2 -H20
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


