Rationalize the observation that when the reaction: is carried out in H 2 ( 18 O), the
Question:
Rationalize the observation that when the reaction:
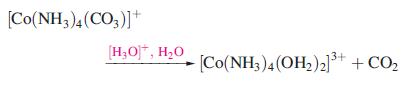
is carried out in H2(18O), the water in the complex contains equal proportions of H2(18O) and H2(16O).
Transcribed Image Text:
[Co(NH3)4 (CO3)]* [H₂O]*, H₂O 13+ [Co(NH3)4 (OH₂)2]³+ + CO₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
The observation can be rationalized based on the concept of the exchange of isotopes during a chemic...View the full answer

Answered By

Aketch Cindy Sunday
I am a certified tutor with over two years of experience tutoring . I have a passion for helping students learn and grow, and I firmly believe that every student has the potential to be successful. I have a wide range of experience working with students of all ages and abilities, and I am confident that I can help students succeed in school.
I have experience working with students who have a wide range of abilities. I have also worked with gifted and talented students, and I am familiar with a variety of enrichment and acceleration strategies.
I am a patient and supportive tutor who is dedicated to helping my students reach their full potential. Thank you for your time and consideration.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When acetone is dissolved in water containing 18O instead of ordinary 16O (i.e., H2 18O instead of H2 16O), the acetone soon begins to acquire 18O and becomes The formation of this oxygen-labeled...
-
An exciting, and often loud, chemical demonstration involves the simple reaction of hydrogen gas and oxygen gas to produce water vapor: 2H2(g) + O2(g) 2H2O(g) The reaction is carried out in soap...
-
The conversion of natural gas, which is mostly methane, into products that contain two or more carbon atoms, such as ethane (C2H6), is a very important industrial chemical process. In principle,...
-
What is the common name for the following structure? CH3 CH3 -C-Br 1 CH3 Isobutyl bromide Osec-Butyl bromide O Neobutyl bromide O Isopropyl methyl bromide t-Butyl bromide
-
Game, LLP, is evaluating the performance of three divisions: Rock, Scissors, and Paper. Using the data that follow, compute the economic value added by each division, and comment on each...
-
Solve each system using the substitution method. If a system is inconsistent or has dependent equations, say so. 5x + y = 2x - 2y = 12 0
-
FUN D IN G A RE TIRE M E N T GO AL. Owen Freeman wishes to have $800,000 in a retirement fund 20 years from now. He can create the retirement fund by making a single lump-sum deposit today. a. If...
-
The emergency room of the community hospital in Farmburg has a receptionist, one doctor, and one nurse. The emergency room opens at time zero, and patients begin to arrive sometime later. Patients...
-
You are working in Walmart as an Accountant as business analyst. You have just been asked to develop a simple information system. Discuss Your Simple information system with the help of diagram? (05...
-
The rate of racemization of [CoL 3 ] where HL = 26.11a is approximately the same as its rate of isomerization into [CoL' 3 ] where HL' =26.11b. What can you deduce about the mechanisms of these...
-
Substitution of H 2 O in [Fe(OH 2 ) 6 ] 3+ by thiocyanate is complicated by proton loss. By considering the reaction scheme below, derive an expression for in terms of the equilibrium and rate...
-
Momentum analysis for a rocket. A rocket has a mass of \(m_{\mathrm{R}}\) and carries fuel with mass \(m_{\mathrm{f}}\) at a given instant of time. Thus the total mass of the system at the current...
-
Brian is considering increasing the length of the cryptographic keys used by his organization. If he adds 8 bits to the encryption key, how many more possible keys will be added to the key space for...
-
Business law SECHON A [100 Marks] Read the scenario below then answer the questions that follow. Contracts are of critical importance especially in daily commercial and business transactions....
-
You may assume that the production costs to the winery are the same for each of the possible wines, despite the differences in volumes with some of the possible wines. Thus maximizing revenue will be...
-
You encounter a split system that uses R-22 refrigerant and observe the following refrigeration parameters from the unit's control display. The unit is operating in cooling mode. Suction pressure:...
-
A refrigerant at -20C is flowing through a 4" schedule 40 carbon steel pipe (inner diameter 102 mm, outer diameter 114 mm); the heat transfer coefficient for the refrigerant is 2500 W/m/K. It is...
-
1. Do Exercise 5-19 using the FIFO method. Note that you first need to calculate the equivalent units of work done in the current period (for direct materials and conversion costs) to complete...
-
Explain why it is not wise to accept a null hypothesis.
-
Propose an efficient synthesis for each of the following transformations: (a) (b) (c) OH
-
Propose an efficient synthesis for each of the following transformations: (a) (b) (c) OH H.
-
Predict the product(s) for each reaction below: (a) (b) (c) (d) * OMe * OMe
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


